Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00078
|
|||||
| Drug Name |
Valacyclovir
|
|||||
| Synonyms |
2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl L-valinate; 2-[(2-amino-6-oxo-3H-purin-9-yl)methoxy]ethyl (2S)-2-amino-3-methylbutanoate; 2-{[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methyl]oxy}ethyl L-valinate; Acyclovir-valine; BW-256U; BW256U87; L-Valine ester with 9-((2-hydroxyethoxy)methyl)guanine; L-Valine, 2-((2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy)ethyl ester; L-valine, 2-[(2-amino-1,6-dihydro-6-oxo-9 H-purin-9-yl)methoxy]ethyl ester, monohydrochloride; TBB067866; Talavir; VACV; ValACV; Valaciclovir (INN); Valaciclovir Hcl; Valaciclovir [INN:BAN]; Valaciclovir, Valtrex; Valacyclover Hydrochloric; Valacyclover Hydrochloride; Valacyclovir; Valacyclovir, (L)-isomer; Valtrex; Valtrex (TN); Virval; Zelitrex; Zelitrex (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Herpes simplex virus infection [ICD11: 1F00] | Approved | [1] | |||
| Shingles [ICD11: 1.00E+91] | Approved | [1] | ||||
| Therapeutic Class |
Antiviral Agents
|
|||||
| Structure |
|
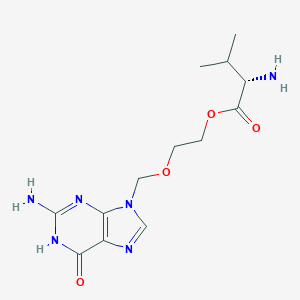 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C13H20N6O4
|
|||||
| Canonical SMILES |
CC(C)C(C(=O)OCCOCN1C=NC2=C1N=C(NC2=O)N)N
|
|||||
| InChI |
InChI=1S/C13H20N6O4/c1-7(2)8(14)12(21)23-4-3-22-6-19-5-16-9-10(19)17-13(15)18-11(9)20/h5,7-8H,3-4,6,14H2,1-2H3,(H3,15,17,18,20)/t8-/m0/s1
|
|||||
| InChIKey |
HDOVUKNUBWVHOX-QMMMGPOBSA-N
|
|||||
| CAS Number |
CAS 124832-27-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 324.34 | Topological Polar Surface Area | 147 | ||
| Heavy Atom Count | 23 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
-0.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103440109
, 104048282
, 104178841
, 104321602
, 117583422
, 118313436
, 12014356
, 124800881
, 126631789
, 126658129
, 126682447
, 128045545
, 131905333
, 134213320
, 134221757
, 134337933
, 135030189
, 135659820
, 137005923
, 137005924
, 142590832
, 14718342
, 14777186
, 14801572
, 14850688
, 14850689
, 14874889
, 15940044
, 160963922
, 162782405
, 163231563
, 163621098
, 29215498
, 43118121
, 46508197
, 47589092
, 48416691
, 50062212
, 56422581
, 57314102
, 626316
, 78076275
, 7980871
, 85279714
, 85789653
, 89736139
, 92309076
, 92710527
, 96025347
, 99775325
|
|||||
| ChEBI ID |
CHEBI:35854
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | ASCT2 | Transporter Info | Alanine/serine/cysteine/threonine transporter 2 | Substrate | [2] | |
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [3] | ||
| PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [4] | ||
| PEPT2 | Transporter Info | Peptide transporter 2 | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OAT3 | Transporter Info | Km = 57.9 microM | Proximal tubule (S2) cells-OAT3 | [3] | |
| PEPT1 | Transporter Info | Km = 1640 microM | Chinese hamster ovary (CHO) cells-PEPT1 | [5] | ||
| PEPT1 | Transporter Info | Km = 2200 microM | Chinese hamster ovary (CHO) cells-PEPT1 | [6] | ||
| PEPT1 | Transporter Info | Km = 2700 microM | Chinese hamster ovary (CHO) cells-PEPT1 | [6] | ||
| PEPT1 | Transporter Info | Km = 5400 microM | Chinese hamster ovary (CHO) cells-PEPT1 | [6] | ||
| PEPT1 | Transporter Info | Km = 6600 microM | Chinese hamster ovary (CHO) cells-PEPT1 | [6] | ||
| PEPT1 | Transporter Info | Km = 7400 microM | Chinese hamster ovary (CHO) cells-PEPT1 | [6] | ||
| PEPT1 | Transporter Info | Km = 2.2 microM | Chinese hamster ovary (CHO) cells-PEPT1 | [7] | ||
| PEPT1 | Transporter Info | Km = 7.4 microM | Chinese hamster ovary (CHO) cells-PEPT1 | [7] | ||
| PEPT1 | Transporter Info | Km = 292 microM | Human enterocyte-like 2 cells (Caco-2)-PEPT1 | [8] | ||
| PEPT1 | Transporter Info | Km = 3.8 microM | Madin-Darby canine kidney (MDCK) cells-PEPT1 | [7] | ||
| PEPT1 | Transporter Info | Km = 5940 microM | Oocytes-PEPT1 | [9] | ||
| PEPT1 | Transporter Info | Km = 5.9 microM | Xenopus oocytes-PEPT1 | [7] | ||
| References | ||||||
| 1 | Acyclovir was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Transport of amino acid-based prodrugs by the Na+- and Cl(-) -coupled amino acid transporter ATB0,+ and expression of the transporter in tissues amenable for drug delivery. J Pharmacol Exp Ther. 2004 Mar;308(3):1138-47. | |||||
| 3 | Human organic anion transporters and human organic cation transporters mediate renal antiviral transport. J Pharmacol Exp Ther. 2002 Mar;300(3):918-24. | |||||
| 4 | Valacyclovir: a substrate for the intestinal and renal peptide transporters PEPT1 and PEPT2. Biochem Biophys Res Commun. 1998 May 19;246(2):470-5. | |||||
| 5 | Interactions of a nonpeptidic drug, valacyclovir, with the human intestinal peptide transporter (hPEPT1) expressed in a mammalian cell line. J Pharmacol Exp Ther. 1999 Apr;289(1):448-54. | |||||
| 6 | Effect of ionization on the variable uptake of valacyclovir via the human intestinal peptide transporter (hPepT1) in CHO cells. Biopharm Drug Dispos. 2000 Jul;21(5):165-74. | |||||
| 7 | Significance of peptide transporter 1 in the intestinal permeability of valacyclovir in wild-type and PepT1 knockout mice. Drug Metab Dispos. 2013 Mar;41(3):608-14. | |||||
| 8 | 5'-Amino acid esters of antiviral nucleosides, acyclovir, and AZT are absorbed by the intestinal PEPT1 peptide transporter. Pharm Res. 1998 Aug;15(8):1154-9. | |||||
| 9 | Direct evidence for peptide transporter (PepT1)-mediated uptake of a nonpeptide prodrug, valacyclovir. Biochem Biophys Res Commun. 1998 Sep 18;250(2):246-51. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
