Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00101
|
|||||
| Drug Name |
Edaravone
|
|||||
| Synonyms |
(MCI-186); 1-Fenyl-3-methyl-2-pyrazolin-5-on; 1-Fenyl-3-methyl-2-pyrazolin-5-on [Czech]; 1-Phenyl-3-methyl-5-oxo-2-pyrazoline; 1-Phenyl-3-methyl-5-pyrazolone; 1-Phenyl-3-methylpyrazolone; 1-Phenyl-3-methylpyrazolone-5; 2,4-Dihydro-5-methyl-2-phenyl-3H-pyrazol-3-one; 3-METHYL-1-PHENYL-2-PYRAZOLIN-5-ONE; 3-Methyl-1-phenyl-2-pyrazoline-5-one; 3-Methyl-1-phenyl-5-pyrazolone; 3-Methyl-1-phenylpyrazol-5-one; 5-Methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one; 5-methyl-2-phenyl-4H-pyrazol-3-one; AE-641/00371017; C.I. Developer 1; CDS1_000986; CI Developer 1; Developer Z; Edarabone; Edaravone; Edaravone (JAN/INN); Edaravone [INN]; Edaravone(jan); IN1263; M0687; MCI 186; Methylphenylpyrazolone; Monopyrazolone; Norantipyrine; Norphenazone; Phenyl methyl pyrazolone; Phenylmethylpyrazolone; Radicut; Radicut (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Amyotrophic lateral sclerosis [ICD11: 8B60.0] | Approved | [1] | |||
| Structure |
|
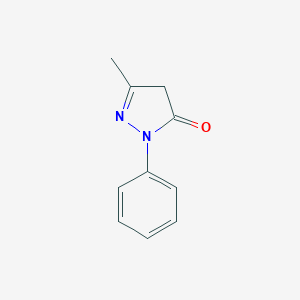 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C10H10N2O
|
|||||
| Canonical SMILES |
CC1=NN(C(=O)C1)C2=CC=CC=C2
|
|||||
| InChI |
InChI=1S/C10H10N2O/c1-8-7-10(13)12(11-8)9-5-3-2-4-6-9/h2-6H,7H2,1H3
|
|||||
| InChIKey |
QELUYTUMUWHWMC-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 89-25-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 174.2 | Topological Polar Surface Area | 32.7 | ||
| Heavy Atom Count | 13 | Rotatable Bond Count | 1 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 2 | |||
| XLogP |
1.3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10537545
, 11113339
, 11120318
, 11120806
, 11121294
, 11121793
, 11122273
, 11336193
, 11337201
, 11361432
, 11362989
, 11363161
, 11365551
, 11365723
, 11368113
, 11368285
, 11371129
, 11371130
, 11371920
, 11373714
, 11374640
, 11376275
, 11376447
, 11384009
, 11462404
, 11485244
, 11489390
, 11490786
, 11492905
, 11494081
, 11512560
, 12014003
, 15219562
, 16959864
, 17389763
, 22389521
, 24885706
, 24897140
, 25622932
, 26612506
, 3133945
, 5663208
, 583397
, 66965
, 69252
, 7848615
, 8150018
, 8152519
, 855774
, 87060
|
|||||
| ChEBI ID |
ChEBI:31530
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OAT1 | Transporter Info | Organic anion transporter 1 | Substrate | [2] | |
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [2] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OAT1 | Transporter Info | Km = 10.8 microM | Human embryonic kidney cells (HEK293)-OAT1 | [2] | |
| OAT3 | Transporter Info | Km = 15.1 microM | Human embryonic kidney cells (HEK293)-OAT3 | [2] | ||
| References | ||||||
| 1 | Edaravone was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Human organic anion transporters 1 (hOAT1/SLC22A6) and 3 (hOAT3/SLC22A8) transport edaravone (MCI-186; 3-methyl-1-phenyl-2-pyrazolin-5-one) and its sulfate conjugate. Drug Metab Dispos. 2007 Aug;35(8):1429-34. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
