Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00197
|
|||||
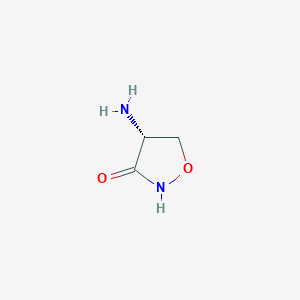
| Drug Name |
Cycloserine
|
|||||
| Synonyms |
(+)-4-Amino-3-isoxazolidinone; (+)-Cycloserine; (4R)-4-Amino-3-isoxazolidinone; (4R)-4-amino-1,2-oxazolidin-3-one; (4R)-4-aminoisoxazolidin-3-one; (R)-(+)-4-Amino-3-isoxazolidinone; (R)-(+)-Cycloserine; (R)-4-AMINO-ISOXAZOLIDIN-3-ONE; (R)-4-Amino-3-isoxazolidinone; (R)-4-Amino-3-isoxazolidone; (R)-Cycloserine; 3-Isoxazolidinone, 4-amino-, (+)-(8CI); 3-Isoxazolidinone, 4-amino-, (4R)-(9CI); 3-Isoxazolidinone, 4-amino-, (R); 3-Isoxazolidinone, 4-amino-, D; Alpha-Cycloserine; C 3909; C-9390; C-9400; Cicloserina; Cicloserina [INN-Spanish]; Cicloserina [Italian]; Closerin; Closina; Cyclo-D-serine; Cyclorin; Cycloserine (JP15/USP/INN); Cycloserine [INN:BAN:JAN]; Cycloserinum; Cycloserinum [INN-Latin]; D-(+)-Cycloserine; D-4-Amino-3-isossazolidone; D-4-Amino-3-isossazolidone [Italian]; D-4-amino-3-isoxazolidinone; D-4-amino-3-isoxazolidone; D-CS; D-amino-3-isoxazolidinone; D-cycloserine; DRG-0195; E-733-A; FA6C7F8B-D080-4EA3-978F-1ECFB5A29D09; Farmiserina; I-1431; K-300; Micoserina; Miroserina; Miroseryn; Novoserin; Orientomycin; Oxamicina; Oxamicina [Italian]; Oxamycin; PA 94; PA-94; R(+)-4-Amino-3-isoxazolidinone; R-(+)-Cycloserine; R-4-Amino-3-isoxazolidinone; RO-1-9213; SC-49088; Seromycin; Seromycin (TN); Tebemicina; Tisomycin; Wasserina
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Dementia [ICD11: 6D8Z] | Approved | [1] | |||
| Tuberculosis [ICD11: 1B1Z] | Approved | [1] | ||||
| Therapeutic Class |
Antiinfective Agents
|
|||||
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C3H6N2O2
|
|||||
| Canonical SMILES |
C1C(C(=O)NO1)N
|
|||||
| InChI |
InChI=1S/C3H6N2O2/c4-2-1-7-5-3(2)6/h2H,1,4H2,(H,5,6)/t2-/m1/s1
|
|||||
| InChIKey |
DYDCUQKUCUHJBH-UWTATZPHSA-N
|
|||||
| CAS Number |
CAS 68-41-7
|
|||||
| Pharmaceutical Properties | Molecular Weight | 102.09 | Topological Polar Surface Area | 64.4 | ||
| Heavy Atom Count | 7 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
-1.5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10257
, 11110913
, 11110914
, 11110915
, 11112194
, 11112195
, 11335273
, 11360512
, 11363754
, 11366316
, 11368878
, 11371441
, 11374029
, 11377040
, 11461484
, 11467117
, 11468237
, 11483744
, 11486765
, 11487900
, 11490187
, 11492120
, 11494674
, 12014545
, 15170469
, 17404815
, 24277962
, 24858076
, 24892907
, 24892979
, 25622405
, 26611680
, 26679210
, 26719637
, 29214856
, 29225230
, 3139717
, 46386864
, 46392863
, 46506865
, 46507135
, 47216632
, 602895
, 7847941
, 7885338
, 7979010
, 8149277
, 8153915
, 829389
, 841629
|
|||||
| ChEBI ID |
ChEBI:40009
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | PAT1 | Transporter Info | Proton-coupled amino acid transporter 1 | Substrate | [2] | |
| PAT2 | Transporter Info | Proton-coupled amino acid transporter 2 | Substrate | [3] | ||
| References | ||||||
| 1 | Cycloserine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Indirect regulation of the intestinal H+-coupled amino acid transporter hPAT1 (SLC36A1). J Cell Physiol. 2005 Aug;204(2):604-13. | |||||
| 3 | Substrate specificity and functional characterisation of the H+/amino acid transporter rat PAT2 (Slc36a2). Br J Pharmacol. 2005 Jan;144(1):28-41. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
