Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00232
|
|||||
| Drug Name |
Probenecid
|
|||||
| Synonyms |
4-((Dipropylamino)sulfonyl)benzoic acid;4-(Di-n-propylsulfamoyl)benzoesaeure; 4-(Dipropylsulfamoyl)benzoic acid; 4-(N,N-Dipropylsulfamoyl)benzoesaeure; 4-[(dipropylamino)sulfonyl]benzoic acid; Apurina; Bencid; Benecid; Benemid; Benemid (TN); Benemide; Benuryl; Benuryl (TN); Biokanol Brand of Probenecid; Col-BENEMID; ColBenemid (co mponent of); ColBenemid (component of); ICN Brand of Probenecid; IDIS Brand of Probenecid; Major Brand of Probenecid; Martec Brand of Probenecid; Merck Brand of Probenecid; Ophthalmic Brand of Probenecid; P-(Dipropylsulfamoyl)benzoic acid; P-(Dipropylsulfamyl)benzoic acid; P-[Dipropylsulfamoyl]benzoic acid; Panuric; Parabenem; Parmed Brand of Probenecid; Polycillin-BRB; Polycillin-PRB (component of); Pro-Cid; Probalan; Probampacin; Probecid; Proben; Probenecid (JP15/USP/INN); Probenecid Major Brand; Probenecid Martec Brand; Probenecid Parmed Brand; Probenecid Weimer; Probenecid Zenith Brand; Probenecid [INN:BAN:JAN]; Probenecid acid; Probenecida; Probenecida [INN-Spanish]; Probenecide; Probenecide [INN-French]; Probenecidum; Probenecidum [INN-Latin]; Probenemid; Probenicid; Probenid; Probexin; Prolongine; Robenecid; Sulprin; Synergid R; Tubophan; Uricosid; Urocid; Valdecasas Brand of Probenecid; Zenith Brand of Probenecid
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Gout [ICD11: FA25] | Approved | [1] | |||
| Hyperuricaemia [ICD11: 5C55.Y] | Approved | [1] | ||||
| Therapeutic Class |
Uricosuric Agents
|
|||||
| Structure |
|
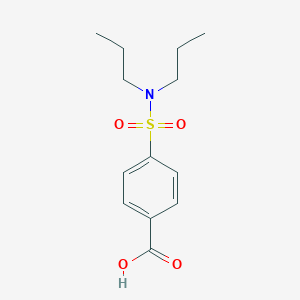 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C13H19NO4S
|
|||||
| Canonical SMILES |
CCCN(CCC)S(=O)(=O)C1=CC=C(C=C1)C(=O)O
|
|||||
| InChI |
InChI=1S/C13H19NO4S/c1-3-9-14(10-4-2)19(17,18)12-7-5-11(6-8-12)13(15)16/h5-8H,3-4,9-10H2,1-2H3,(H,15,16)
|
|||||
| InChIKey |
DBABZHXKTCFAPX-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 57-66-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 285.36 | Topological Polar Surface Area | 83.1 | ||
| Heavy Atom Count | 19 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
3.2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321755
, 10528000
, 11112135
, 11335231
, 11360470
, 11363728
, 11366290
, 11368852
, 11371827
, 11374110
, 11377014
, 11461442
, 11466570
, 11467690
, 11485063
, 11486240
, 11489110
, 11490370
, 11492301
, 11494648
, 14873183
, 17389752
, 24898976
, 26611889
, 26680013
, 26747055
, 26747056
, 26751571
, 29223989
, 3162120
, 46506554
, 47588826
, 47662104
, 47885248
, 47959564
, 47959565
, 48034940
, 48110289
, 48334309
, 48414371
, 48416470
, 48423998
, 5227618
, 7847541
, 7980385
, 8149496
, 8153022
, 81789
, 855953
, 9576
|
|||||
| ChEBI ID |
CHEBI:8426
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MCT6 | Transporter Info | Monocarboxylate transporter 6 | Substrate | [2] | |
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [3] | ||
| OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [4] | ||
| References | ||||||
| 1 | Probenecid was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Quercetin, Morin, Luteolin, and Phloretin Are Dietary Flavonoid Inhibitors of Monocarboxylate Transporter 6. Mol Pharm. 2017 Sep 5;14(9):2930-2936. | |||||
| 3 | MRP2 (ABCC2) transports taxanes and confers paclitaxel resistance and both processes are stimulated by probenecid. Int J Cancer. 2005 Sep 20;116(5):824-9. | |||||
| 4 | Characterization of the efflux transport of 17beta-estradiol-D-17beta-glucuronide from the brain across the blood-brain barrier. J Pharmacol Exp Ther. 2001 Jul;298(1):316-22. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
