Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00248
|
|||||
| Drug Name |
Telmisartan
|
|||||
| Synonyms |
2-[4-[[4-methyl-6-(1-methylbenzimidazol-2-yl)-2-propylbenzimidazol-1-yl]methyl]phenyl]benzoic acid; 4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidazol)-1'-yl)methyl)-(1,1'-biphenyl)-2-carboxylic acid; 4'-((4-Methyl-6-(1-methyl-2-benzimidazolyl)-2-propyl-1-benzimidazolyl)methyl)-2-biphenylcarboxylic acid; 4'-((4-mehtyl-6-(1-methyl-2-benzimidazolyl)-2-propyl-1-benzimmidazolyl)methyl)-2-biphenylcarboxylic acid; 4'-[(1,4'-dimethyl-2'propyl[2,6'-bi-1H-benzimidazol]-1'-yl)methyl]-[1,1'-biphenyl]-2-carboxylic acid; 4'-[(1,7'-dimethyl-2'-propyl-1H,3'H-2,5'-bibenzimidazol-3'-yl)methyl][1,1'-biphenyl]-2-carboxylic acid; 4'-[(1,7'-dimethyl-2'-propyl-1H,3'H-2,5'-bibenzimidazol-3'-yl)methyl]biphenyl-2-carboxylic acid; Abbott brand of telmisartan; BIBR 277; BIBR 277SE; BIBR-277; BIBR-277-SE; BIBR-277SE; Bay 68-9291; Boehringer Ingelheim brand of telmisartan; Glaxo Wellcome brand of telmisartan; GlaxoSmithKline brand of telmisartan; Kinzal; Kinzalmono; Micardis; Micardis (TN); Micardis, Targit, Temax, BIBR277, Telmisartan; Pritor; Telmisartan (JAN/USAN/INN); Telmisartan [USAN:INN]; YM-086
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | High blood pressure [ICD11: BA00] | Approved | [1] | |||
| Therapeutic Class |
Antihypertensive Agents
|
|||||
| Structure |
|
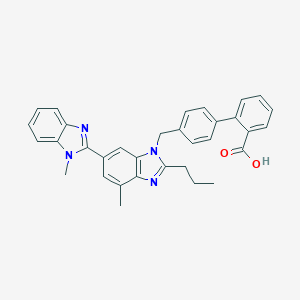 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C33H30N4O2
|
|||||
| Canonical SMILES |
CCCC1=NC2=C(N1CC3=CC=C(C=C3)C4=CC=CC=C4C(=O)O)C=C(C=C2C)C5=NC6=CC=CC=C6N5C
|
|||||
| InChI |
InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39)
|
|||||
| InChIKey |
RMMXLENWKUUMAY-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 144701-48-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 514.6 | Topological Polar Surface Area | 72.9 | ||
| Heavy Atom Count | 39 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 4 | |||
| XLogP |
6.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103271997
, 103974749
, 104253350
, 104335216
, 11372057
, 11374980
, 11485867
, 11489792
, 11490904
, 11493075
, 11528632
, 117546129
, 117867825
, 12014783
, 14860450
, 26612858
, 26680963
, 26719833
, 26749005
, 26749006
, 43122721
, 46386620
, 46505370
, 46530547
, 47350860
, 47796474
, 48416595
, 49648460
, 49830991
, 50107502
, 56312042
, 56313977
, 57315885
, 6866718
, 7847693
, 79018553
, 7980737
, 81041072
, 81092854
, 8190092
, 85209733
, 85789678
, 87225392
, 92124871
, 92308092
, 92308546
, 92309313
, 92712346
, 93166523
, 9912
|
|||||
| ChEBI ID |
ChEBI:9434
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [2] | |
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [2] | ||
| OATP2B1 | Transporter Info | Organic anion transporting polypeptide 2B1 | Substrate | [3] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OATP1B3 | Transporter Info | Km = 0.81 microM | Human embryonic kidney cells (HEK293)-OATP1B3 | [2] | |
| OATP1B3 | Transporter Info | Km = 3.4 microM | Human embryonic kidney cells (HEK293)-OATP1B3 | [3] | ||
| OATP2B1 | Transporter Info | Km = 1.09 microM | Human embryonic kidney cells (HEK293)-OATP2B1 | [3] | ||
| References | ||||||
| 1 | Telmisartan was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Predominant contribution of OATP1B3 to the hepatic uptake of telmisartan, an angiotensin II receptor antagonist, in humans. Drug Metab Dispos. 2006 Jul;34(7):1109-15. | |||||
| 3 | Establishment of a set of double transfectants coexpressing organic anion transporting polypeptide 1B3 and hepatic efflux transporters for the characterization of the hepatobiliary transport of telmisartan acylglucuronide. Drug Metab Dispos. 2008 Apr;36(4):796-805. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
