Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00262
|
|||||
| Drug Name |
Sirolimus
|
|||||
| Synonyms |
(-)-Rapamycin; 23,27-Epoxy-3H-pyrido(2,1-c)(1,4)oxaazacyclohentriacontine; 23,27-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine; 23,27-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine-1,5,11,28,29; 3H-pyrido(2,1-c)(1,4)oxaazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone; AY 22989; AY-22989; Antibiotic AY 22989; DE-109; Heptadecahydro-9,27-dihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-hydroxy; LCP-Siro; MS-R001; Perceiva; RAP; RAPA; RPM; Rapammune; Rapamune; Rapamune (TN); Rapamycin; Rapamycin (TN); Rapamycin C-7, analog 4; Rapamycin Immunosuppressant Drug; Rapamycin from Streptomyces hygroscopicus; SIIA 9268A; SILA 9268A; Sirolimus (MTOR inhibitor); Sirolimus (RAPAMUNE); Sirolimus (USAN/INN); Sirolimus [USAN:BAN:INN]; Sirolimus, Rapamune,Rapamycin; WY-090217; Wy 090217
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Organ transplant rejection [ICD11: NE84] | Approved | [1] | |||
| Therapeutic Class |
Immunosuppressive Agents
|
|||||
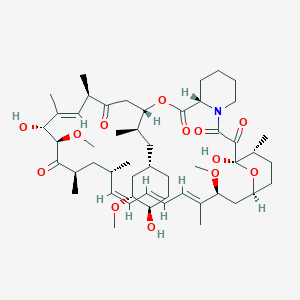
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C51H79NO13
|
|||||
| Canonical SMILES |
CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)O)C)C)O)OC)C)C)C)OC
|
|||||
| InChI |
InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1
|
|||||
| InChIKey |
QFJCIRLUMZQUOT-HPLJOQBZSA-N
|
|||||
| CAS Number |
CAS 53123-88-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 914.2 | Topological Polar Surface Area | 195 | ||
| Heavy Atom Count | 65 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 13 | |||
| XLogP |
6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
11110132
, 11110134
, 11110139
, 11528741
, 12014635
, 14816423
, 14816426
, 24430811
, 24899339
, 26701762
, 26705530
, 26705537
, 26709840
, 26713306
, 37101833
, 46391895
, 46392864
, 47289337
, 48416541
, 49635682
, 49815675
, 50103901
, 50139515
, 56310631
, 56310880
, 56311583
, 56311585
, 56311714
, 56311827
, 56311949
, 56311983
, 56312137
, 56312183
, 56312184
, 56312185
, 56312186
, 56312645
, 56312887
, 56313330
, 56313354
, 56313375
, 56313498
, 56313803
, 56313974
, 56314022
, 636884
, 7890221
, 7982025
, 8145920
, 830671
|
|||||
| ChEBI ID |
ChEBI:9168
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Sirolimus was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Pharmacokinetic and pharmacodynamic interactions between the immunosuppressant sirolimus and the lipid-lowering drug ezetimibe in healthy volunteers. Clin Pharmacol Ther. 2010 Jun;87(6):663-7. | |||||
| 3 | Pharmacogenetics of tacrolimus and sirolimus in renal transplant patients: from retrospective analyses to prospective studies. Transplant Proc. 2007 Sep;39(7):2142-4. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
