Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00266
|
|||||
| Drug Name |
L-phenylalanine
|
|||||
| Synonyms |
(2S)-2-amino-3-phenylpropanoic acid; (L)-Phenylalanine; (S)-(-)-Phenylalanine; (S)-2-Amino-3-phenylpropanoic acid; (S)-2-Amino-3-phenylpropionic acid; (S)-Phenylalanine; (S)-alpha-Amino-benzenepropanoic acid; (S)-alpha-Amino-beta-phenylpropionic acid; (S)-alpha-Aminohydrocinnamic acid; 1F9436B3-8B0D-4AC6-A004-4249B0BDA436; 3-Phenyl-L-alanine; 3-Phenylalanine; 3-[4-[bis(2-chloroethyl)amino]phenyl]-2-formamidopropanoic acid; Alpha-Aminohydrocinnamic acid; Antibiotic FN 1636; Beta-Phenyl-L-alanine; Beta-Phenylalanine; CB 3208; Endophenyl; Fenilalanina; Fenilalanina [Spanish]; H-Phe-OH; L-3-(p-(Bis(2-chloroethyl)amino)phenyl)-N-formylalanine; L-Antibiotic FN 1636; L-PHENYL ALANINE (SEE ALSO 22839-47-0, ASPARTAME; L-PHENYLALININE; L-Phenylalanine (JP15); L-Phenylalanine, 4-(bis(2-chloroethyl)amino)-N-formyl-(9CI); N-Formyl-L-p-sarcolysin; N-Formyl-L-sarcolysin; N-Formylmelphalan; NCI9959; Phe; Phenylalanine; Phenylalanine (USP/INN); Phenylalanine (VAN); Phenylalanine [USAN:INN:JAN]; Phenylalaninum; Phenylalaninum [Latin]
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Dietary shortage [ICD11: 5B7Z] | Approved | [1] | |||
| Therapeutic Class |
Dietary supplement
|
|||||
| Structure |
|
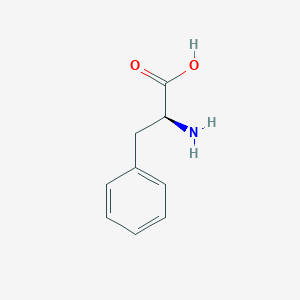 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C9H11NO2
|
|||||
| Canonical SMILES |
C1=CC=C(C=C1)CC(C(=O)O)N
|
|||||
| InChI |
InChI=1S/C9H11NO2/c10-8(9(11)12)6-7-4-2-1-3-5-7/h1-5,8H,6,10H2,(H,11,12)/t8-/m0/s1
|
|||||
| InChIKey |
COLNVLDHVKWLRT-QMMMGPOBSA-N
|
|||||
| CAS Number |
CAS 63-91-2
|
|||||
| Pharmaceutical Properties | Molecular Weight | 165.19 | Topological Polar Surface Area | 63.3 | ||
| Heavy Atom Count | 12 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
-1.5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10531434
, 11457628
, 11459655
, 11528323
, 11532528
, 14710668
, 15219430
, 24770335
, 24770338
, 24770342
, 24887247
, 24898274
, 24898657
, 24898971
, 24901797
, 26651814
, 26702643
, 26748791
, 26748792
, 26753740
, 29225143
, 3134198
, 3379
, 4252550
, 46391851
, 46393329
, 46505708
, 50107465
, 57323218
, 57578102
, 57652717
, 57654567
, 57655097
, 584667
, 6436530
, 78164833
, 7847089
, 7889851
, 81044023
, 81044025
, 81044569
, 81067307
, 81067312
, 8144203
, 8153851
, 822460
, 824902
, 832928
, 838754
, 841094
|
|||||
| ChEBI ID |
ChEBI:17295
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | LAT2 | Transporter Info | L-type amino acid transporter 2 | Substrate | [2] | |
| LAT3 | Transporter Info | L-type amino acid transporter 3 | Substrate | [3] | ||
| LAT4 | Transporter Info | L-type amino acid transporter 4 | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | LAT2 | Transporter Info | Km = 12.2 microM | Xenopus oocytes-LAT2 | [2] | |
| References | ||||||
| 1 | L-phenylalanine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Quantifying the relative contributions of different solute carriers to aggregate substrate transport. Sci Rep. 2017 Jan 16;7:40628. | |||||
| 3 | Identification of a novel system L amino acid transporter structurally distinct from heterodimeric amino acid transporters. J Biol Chem. 2003 Oct 31;278(44):43838-45. | |||||
| 4 | Anticipation of food intake induces phosphorylation switch to regulate basolateral amino acid transporter LAT4 (SLC43A2) function. J Physiol. 2019 Jan;597(2):521-542. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
