Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00286
|
|||||
| Drug Name |
Benazepril
|
|||||
| Synonyms |
2-[(3S)-3-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]-2-oxo-4,5-dihydro-3H-1-benzazepin-1-yl]acetic acid; Benazapril; Benazepril (INN); Benazepril Sandoz; Benazepril Sandoz (TN); Benazepril [INN:BAN]; Benazeprilum; Benazeprilum [Latin]; Benzazepril; CGS-14824-A; Forteekor [veterinary]; Forteekor [veterinary] (TN); Fortekor (TN); Lotensin (TN); [(3S)-3-({(1S)-1-[(ethyloxy)carbonyl]-3-phenylpropyl}amino)-2-oxo-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]acetic acid; [(3S)-3-{[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino}-2-oxo-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]acetic acid; [(3S)-3-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}-2-oxo-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]acetic acid
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | High blood pressure [ICD11: BA00] | Approved | [1] | |||
| Therapeutic Class |
Antihypertensive Agents
|
|||||
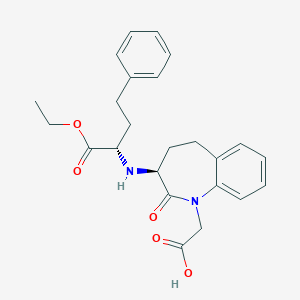
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C24H28N2O5
|
|||||
| Canonical SMILES |
CCOC(=O)C(CCC1=CC=CC=C1)NC2CCC3=CC=CC=C3N(C2=O)CC(=O)O
|
|||||
| InChI |
InChI=1S/C24H28N2O5/c1-2-31-24(30)20(14-12-17-8-4-3-5-9-17)25-19-15-13-18-10-6-7-11-21(18)26(23(19)29)16-22(27)28/h3-11,19-20,25H,2,12-16H2,1H3,(H,27,28)/t19-,20-/m0/s1
|
|||||
| InChIKey |
XPCFTKFZXHTYIP-PMACEKPBSA-N
|
|||||
| CAS Number |
CAS 86541-75-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 424.5 | Topological Polar Surface Area | 95.9 | ||
| Heavy Atom Count | 31 | Rotatable Bond Count | 10 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
1.3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103220490
, 103931866
, 11364868
, 11367430
, 11369992
, 11372806
, 11373951
, 11378161
, 114149713
, 11484079
, 11488298
, 11491489
, 11492101
, 11495736
, 11533499
, 11654004
, 117871221
, 124893187
, 126663871
, 127334967
, 127334968
, 127334969
, 127334970
, 127334971
, 127334972
, 127334973
, 127334974
, 127334975
, 127334976
, 127334977
, 127334978
, 129720750
, 131328443
, 14856184
, 14880498
, 29217496
, 39384400
, 46507884
, 47291378
, 47960005
, 48415605
, 49877926
, 50111699
, 50171976
, 51091824
, 53788310
, 85787982
, 9061
, 93166958
, 94568986
|
|||||
| ChEBI ID |
ChEBI:3011
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [2] | |
| PEPT2 | Transporter Info | Peptide transporter 2 | Substrate | [2] | ||
| References | ||||||
| 1 | Benazepril was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Transport of angiotensin-converting enzyme inhibitors by H+/peptide transporters revisited. J Pharmacol Exp Ther. 2008 Nov;327(2):432-41. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
