Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00292
|
|||||
| Drug Name |
Procainamide
|
|||||
| Synonyms |
2-Diethylaminoethylamid kyseliny p-aminobenzoove; 2-Diethylaminoethylamid kyseliny p-aminobenzoove [Czech]; 4-Amino-N-(2-(Diethylamino)Ethyl)Benzamide Sulfate; 4-Amino-N-(2-(diethylamino)ethyl)benzamide; 4-Amino-N-[2-(diethylamino)ethyl]benzamide; 4-amino-N-(2-diethylaminoethyl)-benzamide; 4-amino-N-(2-diethylaminoethyl)benzamide; Benzamide, 4-amino-N-(2-(diethylamino)ethyl)-(9CI); Biocoryl; Novocainamid; Novocainamide; Novocaine amide; Novocamid; P-Amino-N-(2-diethylaminoethyl)benzamide; P-Aminobenzoic diethylaminoethylamide; Procainamida; Procainamida [INN-Spanish]; Procainamide (INN); Procainamide [INN:BAN]; Procainamidum; Procainamidum [INN-Latin]; Procaine amide; Procamide; Procan; Procan (TN); Procanbid; Procanbid (TN); Procapan; Procapan (free base); Pronestyl; Pronestyl (TN); Pronestyl-Sr
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Ventricular arrhythmias [ICD11: BC71] | Approved | [1] | |||
| Therapeutic Class |
Antiarrhythmic Agents
|
|||||
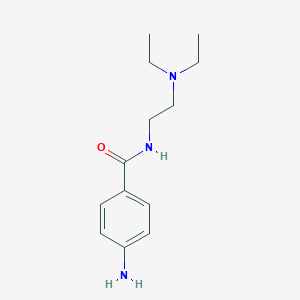
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C13H21N3O
|
|||||
| Canonical SMILES |
CCN(CC)CCNC(=O)C1=CC=C(C=C1)N
|
|||||
| InChI |
InChI=1S/C13H21N3O/c1-3-16(4-2)10-9-15-13(17)11-5-7-12(14)8-6-11/h5-8H,3-4,9-10,14H2,1-2H3,(H,15,17)
|
|||||
| InChIKey |
REQCZEXYDRLIBE-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 51-06-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 235.33 | Topological Polar Surface Area | 58.4 | ||
| Heavy Atom Count | 17 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
0.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10524666
, 11111690
, 11111691
, 11120271
, 11120759
, 11121247
, 11121528
, 11122008
, 11132695
, 11336106
, 11361345
, 11362597
, 11363730
, 11365159
, 11366292
, 11367721
, 11368854
, 11370465
, 11370466
, 11371829
, 11373322
, 11374112
, 11375883
, 11377016
, 11382660
, 11462317
, 11466365
, 11467485
, 11485065
, 11486030
, 11489111
, 11490371
, 11492302
, 11494650
, 15220788
, 26751611
, 26751612
, 29223991
, 3138733
, 3296200
, 46507313
, 47216907
, 47440400
, 47662439
, 47810893
, 5227620
, 7980387
, 8153024
, 87927
, 9605
|
|||||
| ChEBI ID |
ChEBI:8428
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MATE1 | Transporter Info | Multidrug and toxin extrusion protein 1 | Substrate | [2] | |
| MATE2 | Transporter Info | Multidrug and toxin extrusion protein 2 | Substrate | [2] | ||
| OCT-1 | Transporter Info | Organic cation transporter 1 | Substrate | [3] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | MATE1 | Transporter Info | Km = 1230 microM | Human embryonic kidney cells (HEK293)-MATE1 | [2] | |
| MATE2 | Transporter Info | Km = 1580 microM | Human embryonic kidney cells (HEK293)-MATE2K | [2] | ||
| MATE2 | Transporter Info | Km = 4100 microM | Human embryonic kidney cells (HEK293)-MATE2K | [4] | ||
| References | ||||||
| 1 | Procainamide was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol. 2007 Jul 15;74(2):359-71. | |||||
| 3 | Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem. 2013 Sep 26;56(18):7232-42. | |||||
| 4 | Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J Am Soc Nephrol. 2006 Aug;17(8):2127-35. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
