Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00320
|
|||||
| Drug Name |
Ceftizoxime
|
|||||
| Synonyms |
(6R,7R)-7-(2-(2-Amino-4-thiazolyl)-2Z-(methoxyimino)acetamido)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-2-carbonsaeure; (6R,7R)-7-(2-(2-Amino-4-thiazolyl)glyoxyamido)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-carbonsaeure-7-(Z)-(O-methyloxim); (6R,7R)-7-({(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-[(methyloxy)imino]acetyl}amino)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7beta-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-2,3-didehydropenam-2-carboxylic acid; Cefizox (TN); Ceftix; Ceftizoxima; Ceftizoxima[INN-Spanish]; Ceftizoxime (INN); Ceftizoxime Monosodium Salt; Ceftizoxime [INN:BAN]; Ceftizoximum; Ceftizoximum [INN-Latin]; Eposerin; FK-749; FK749; FR 13749; FR-13479; FR-13749; SK&F 88373-2; SKF-88373; Syn-7-(2-(2-Amino-4-thiazolyl)-2-methoxyiminoacetamido)-3-cephem-4-carboxylic acid
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Gram-positive & negative bacteria infections [ICD11: 1A00-1H0Z] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
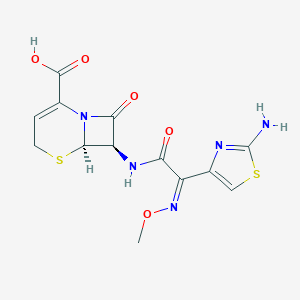 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C13H13N5O5S2
|
|||||
| Canonical SMILES |
CON=C(C1=CSC(=N1)N)C(=O)NC2C3N(C2=O)C(=CCS3)C(=O)O
|
|||||
| InChI |
InChI=1S/C13H13N5O5S2/c1-23-17-7(5-4-25-13(14)15-5)9(19)16-8-10(20)18-6(12(21)22)2-3-24-11(8)18/h2,4,8,11H,3H2,1H3,(H2,14,15)(H,16,19)(H,21,22)/b17-7-/t8-,11-/m1/s1
|
|||||
| InChIKey |
NNULBSISHYWZJU-LLKWHZGFSA-N
|
|||||
| CAS Number |
CAS 68401-81-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 383.4 | Topological Polar Surface Area | 201 | ||
| Heavy Atom Count | 25 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 10 | |||
| XLogP |
0
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103174922
, 114606946
, 11641637
, 124766423
, 131323579
, 134222601
, 134337748
, 135009953
, 137006383
, 140396425
, 160870913
, 160964629
, 162178763
, 170505879
, 175268604
, 179150929
, 223678791
, 226423279
, 249865495
, 251912547
, 251916656
, 252817575
, 43181664
, 46505647
, 48415732
, 49847130
, 50050951
, 50236104
, 51091962
, 57370867
, 602886
, 7978886
, 87256558
, 9107
|
|||||
| ChEBI ID |
CHEBI:553473
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MRP4 | Transporter Info | Multidrug resistance-associated protein 4 | Substrate | [2] | |
| OAT1 | Transporter Info | Organic anion transporter 1 | Substrate | [3] | ||
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | MRP4 | Transporter Info | Km = 18 microM | Human embryonic kidney cells (HEK293)-MRP4 | [2] | |
| References | ||||||
| 1 | Ceftizoxime was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Oral availability of cefadroxil depends on ABCC3 and ABCC4. Drug Metab Dispos. 2012 Mar;40(3):515-21. | |||||
| 3 | FDA Drug Development and Drug Interactions | |||||
| 4 | Human organic anion transporter hOAT3 is a potent transporter of cephalosporin antibiotics, in comparison with hOAT1. Biochem Pharmacol. 2005 Oct 1;70(7):1104-13. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
