Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00404
|
|||||
| Drug Name |
Valganciclovir
|
|||||
| Synonyms |
5-Amino-3-[1-(hydroxymethyl)-2-(L-valyloxy)ethoxymethyl]-6,7-dihydro-3H-imidazo[4,5-d]pyrimidin-7-one; Cymeval; L-Valine, 2-((2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy)-3-hydroxypropyl ester; L-Valine, ester with ganciclovir; RO1079070/194; RS 79070; Valcyte (TN); Valganciclovir (INN); Valganciclovir (Oral); Valganciclovir [INN:BAN]; [2-[(2-amino-6-oxo-3H-purin-9-yl)methoxy]-3-hydroxypropyl] (2S)-2-amino-3-methylbutanoate
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Cytomegalovirus infections [ICD11: 1D82] | Approved | [1] | |||
| Therapeutic Class |
Antiviral Agents
|
|||||
| Structure |
|
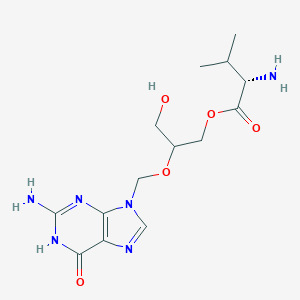 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C14H22N6O5
|
|||||
| Canonical SMILES |
CC(C)C(C(=O)OCC(CO)OCN1C=NC2=C1N=C(NC2=O)N)N
|
|||||
| InChI |
InChI=1S/C14H22N6O5/c1-7(2)9(15)13(23)24-4-8(3-21)25-6-20-5-17-10-11(20)18-14(16)19-12(10)22/h5,7-9,21H,3-4,6,15H2,1-2H3,(H3,16,18,19,22)/t8?,9-/m0/s1
|
|||||
| InChIKey |
WPVFJKSGQUFQAP-GKAPJAKFSA-N
|
|||||
| CAS Number |
CAS 175865-60-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 354.36 | Topological Polar Surface Area | 167 | ||
| Heavy Atom Count | 25 | Rotatable Bond Count | 9 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
-1.5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
104330216
, 117576840
, 123099361
, 126681567
, 129616655
, 134337936
, 135022474
, 135611114
, 137260967
, 141634799
, 142044094
, 14852135
, 14852136
, 152035502
, 152105972
, 15916891
, 160647806
, 160964847
, 162205159
, 16292870
, 163621140
, 164830911
, 16867892
, 172086164
, 178101427
, 184546497
, 184823771
, 223661894
, 226416180
, 237715327
, 251916051
, 251917399
, 43121054
, 46505524
, 47205808
, 50064546
, 50113281
, 50647485
, 57315041
, 615108
, 7980872
, 91616537
, 92251598
|
|||||
| ChEBI ID |
CHEBI:63635
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | ASCT2 | Transporter Info | Alanine/serine/cysteine/threonine transporter 2 | Substrate | [2] | |
| PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Valganciclovir was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Transport of amino acid esters and the amino-acid-based prodrug valganciclovir by the amino acid transporter ATB(0,+). Pharm Res. 2004 Jul;21(7):1303-10. | |||||
| 3 | Transport of valganciclovir, a ganciclovir prodrug, via peptide transporters PEPT1 and PEPT2. J Pharm Sci. 2000 Jun;89(6):781-9. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
