Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00446
|
|||||
| Drug Name |
Repaglinide
|
|||||
| Synonyms |
(+)-2-Ethoxy-alpha-(((S)-alpha-isobutyl-o-piperidinobenzyl)carbamoyl)-p-toluic acid; (S)-(+)-2-Ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonylmethyl]benzoic acid; (S)-2-Ethoxy-4-(2-((methyl-1-(2-(1-piperidinyl)phenyl)butyl)amino)-2-oxoethyl)-benzoic acid; (S)-2-ethoxy-4-(2-((3-methyl-1-(2-(1-piperidinyl)-phenyl)butyl)amino)-2-oxoethyl)-benzoic acid; 111GE012; 2-ethoxy-4-(2-((3-methyl-1-(2-(1-piperidinyl)phenyl)butyl)amino)-2-oxoethyl)benzoic acid; 2-ethoxy-4-(2-{[(1S)-3-methyl-1-(2-piperidin-1-ylphenyl)butyl]amino}-2-oxoethyl)benzoic acid; 2-ethoxy-4-[2-({(1S)-3-methyl-1-[2-(piperidin-1-yl)phenyl]butyl}amino)-2-oxoethyl]benzoic acid; 2-ethoxy-4-[2-[[(1S)-3-methyl-1-(2-piperidin-1-ylphenyl)butyl]amino]-2-oxoethyl]benzoic acid; 2-ethoxy-N-(alpha-(2-methyl-1-propyl)-2-piperidinobenzyl)-4-carbamoylmethylbenzoic acid; AG-EE 388; AG-EE 388 ZW; AG-EE 623 ZW; AG-EE-388; AG-EE-623 ZW; AGEE-623ZW; Actulin; Glaxo Wellcome brand of replaginide; GlucoNorm; GlucoNorm (TN); NN-623; Novo Nordisk brand 2 of repaglinide; Novo Nordisk brand of repaglinide; NovoNorm; NovoNorm (TN); Prandin; Prandin (TN); Prandin, GlucoNorm, NovoNorm, Repaglinide; Repa-glinide; Repaglinida; Repaglinida [INN-Spanish]; Repaglinide (JAN/USP/INN); Repaglinide [USAN]; Repaglinide, (+-)-isomer; Repaglinidum; Repaglinidum [INN-Latin]; SMP-508
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Type 2 diabetes [ICD11: 5A11] | Approved | [1] | |||
| Therapeutic Class |
Hypoglycemic Agents
|
|||||
| Structure |
|
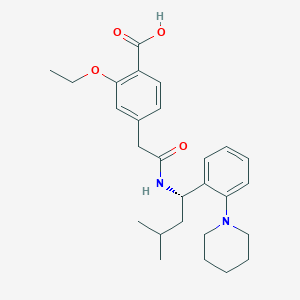 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C27H36N2O4
|
|||||
| Canonical SMILES |
CCOC1=C(C=CC(=C1)CC(=O)NC(CC(C)C)C2=CC=CC=C2N3CCCCC3)C(=O)O
|
|||||
| InChI |
InChI=1S/C27H36N2O4/c1-4-33-25-17-20(12-13-22(25)27(31)32)18-26(30)28-23(16-19(2)3)21-10-6-7-11-24(21)29-14-8-5-9-15-29/h6-7,10-13,17,19,23H,4-5,8-9,14-16,18H2,1-3H3,(H,28,30)(H,31,32)/t23-/m0/s1
|
|||||
| InChIKey |
FAEKWTJYAYMJKF-QHCPKHFHSA-N
|
|||||
| CAS Number |
CAS 135062-02-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 452.6 | Topological Polar Surface Area | 78.9 | ||
| Heavy Atom Count | 33 | Rotatable Bond Count | 10 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
5.2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103394824
, 103914669
, 104041348
, 104179029
, 104335174
, 11112894
, 11467074
, 11468194
, 11486642
, 117545849
, 117873275
, 12014667
, 121363609
, 124659000
, 124757239
, 124799919
, 125164043
, 14808699
, 14833410
, 24724596
, 26719812
, 43122707
, 46386564
, 46508150
, 47425572
, 48244693
, 48319772
, 48395177
, 48416511
, 49648522
, 49699266
, 49888451
, 50100536
, 52108264
, 53789672
, 57315871
, 7847660
, 7980507
, 81092844
, 8190080
, 85788088
, 92125938
, 92308165
, 92308498
, 92719265
, 93166496
, 9872
, 99218180
, 99431925
, 99437146
|
|||||
| ChEBI ID |
CHEBI:8805
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [2] | |
| References | ||||||
| 1 | Repaglinide was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Disease-Associated Changes in Drug Transporters May Impact the Pharmacokinetics and/or Toxicity of Drugs: A White Paper From the International Transporter Consortium. Clin Pharmacol Ther. 2018 Nov;104(5):900-915. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
