Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00464
|
|||||
| Drug Name |
Anastrozole
|
|||||
| Synonyms |
1,3-benzenediacetonitrile, a, a,a', a'-tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl); 2,2'-(5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-phenylene)bis(2-methylpropionitrile); 2,2'-[5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-phenylene]bis(2-methylpropanenitrile); 2,2'-[5-(1H-1,2,4-triazol-1-ylmethyl)benzene-1,3-diyl]bis(2-methylpropanenitrile); 2-[3-(2-cyanopropan-2-yl)-5-(1,2,4-triazol-1-ylmethyl)phenyl]-2-methylpropanenitrile; Alpha,alpha,alpha',alpha'-Tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl)-m-benzenediacetonitrile; Alpha,alpha,alpha',alpha'-tetramethyl-5(1H-1,2,4-triazol-1-ylmethyl)-m-benzenediacetonitrile; Anastrazole; Anastrole; Anastrozol; Anastrozole (JAN/USAN/INN); Anastrozole [USAN:INN:BAN]; Arimidex; Arimidex (TN); Arimidex (Zeneca); Arimidex, Anastrozole; Asiolex; Astra brand of anastrozole; AstraZeneca brand of anastrozole; ZD 1033; ZD-1033; ZD1033; Zeneca ZD 1033; Zeneca brand of anastrozole
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Breast cancer [ICD11: 2C60-2C6Z] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
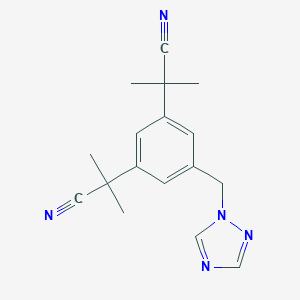
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C17H19N5
|
|||||
| Canonical SMILES |
CC(C)(C#N)C1=CC(=CC(=C1)CN2C=NC=N2)C(C)(C)C#N
|
|||||
| InChI |
InChI=1S/C17H19N5/c1-16(2,9-18)14-5-13(8-22-12-20-11-21-22)6-15(7-14)17(3,4)10-19/h5-7,11-12H,8H2,1-4H3
|
|||||
| InChIKey |
YBBLVLTVTVSKRW-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 120511-73-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 293.4 | Topological Polar Surface Area | 78.3 | ||
| Heavy Atom Count | 22 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 4 | |||
| XLogP |
2.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103478231
, 10359
, 104299862
, 11528617
, 117672710
, 118313740
, 12014321
, 124659088
, 124757065
, 124801312
, 125163869
, 125311691
, 125339200
, 126630179
, 126657740
, 126667645
, 129769972
, 131296284
, 134337917
, 135018130
, 135698190
, 135727309
, 136348802
, 14751420
, 26719808
, 26758041
, 29221365
, 46386543
, 46504987
, 46510669
, 48415567
, 49681575
, 5128740
, 53007586
, 535026
, 53789173
, 56311273
, 56313558
, 56313576
, 57321190
, 58106884
, 7848023
, 7978492
, 81040864
, 8151486
, 87351875
, 92308653
, 92308946
, 92712142
, 99436928
|
|||||
| ChEBI ID |
CHEBI:2704
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | SNAT7 | Transporter Info | Putative sodium-coupled neutral amino acid transporter 7 | Substrate | [2] | |
| References | ||||||
| 1 | Anastrozole was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Anastrozole Aromatase Inhibitor Plasma Drug Concentration Genome-Wide Association Study: Functional Epistatic Interaction Between SLC38A7 and ALPPL2. Clin Pharmacol Ther. 2019 Jan 16. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
