Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00507
|
|||||
| Drug Name |
Lomefloxacin
|
|||||
| Synonyms |
(+-)-1-Ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid; 1,4-Dihydro-6,8-difluoro-1-ethyl-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid; 1-Ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid; 1-ethyl-6,8-difluoro-7-(3-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid; 1-ethyl-6,8-difluoro-7-(3-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid; 3-Quinolinecarboxylic acid, 1-ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-1-piperazinyl)-4-oxo-, monohydrochloride; DM 10 (bactericide); DM-10; LFLX; Lomefloxacin (USAN); Lomefloxacin [USAN:BAN:INN]; Lomefloxacine; Lomefloxacine [French]; Lomefloxacino; Lomefloxacino [Spanish]; Lomefloxacinum; Lomefloxacinum[Latin]; Maxaquin (TN); Maxaquin (hydrochloride); NY-198 (hydrochloride); Okacyn (TN); SC 4711; SC 47111A; SC-4711; SC-47111 (hydrochloride); SC-47111A; SC-47111B (mesylate); Uniquin (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Bacterial infections [ICD11: 1A00-1H0Z] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
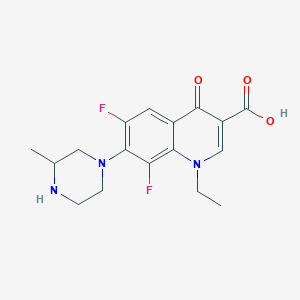 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C17H19F2N3O3
|
|||||
| Canonical SMILES |
CCN1C=C(C(=O)C2=CC(=C(C(=C21)F)N3CCNC(C3)C)F)C(=O)O
|
|||||
| InChI |
InChI=1S/C17H19F2N3O3/c1-3-21-8-11(17(24)25)16(23)10-6-12(18)15(13(19)14(10)21)22-5-4-20-9(2)7-22/h6,8-9,20H,3-5,7H2,1-2H3,(H,24,25)
|
|||||
| InChIKey |
ZEKZLJVOYLTDKK-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 98079-51-7
|
|||||
| Pharmaceutical Properties | Molecular Weight | 351.35 | Topological Polar Surface Area | 72.9 | ||
| Heavy Atom Count | 25 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
-0.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103178831
, 11335540
, 11360779
, 11364325
, 11366887
, 11369449
, 11372706
, 11373775
, 11377611
, 11461751
, 11466266
, 11467386
, 11484587
, 11485972
, 11488512
, 11491309
, 11491973
, 11495245
, 14851991
, 24278514
, 29223062
, 46508499
, 47291062
, 47588922
, 47588923
, 47736397
, 47736398
, 48035031
, 48110376
, 48334411
, 48334412
, 49698401
, 49835841
, 50042514
, 50111115
, 50122921
, 50122922
, 50203310
, 5037068
, 56313756
, 57322062
, 602955
, 7849377
, 7979788
, 8152478
, 85321506
, 85788434
, 90340951
, 92309292
, 9289
|
|||||
| ChEBI ID |
CHEBI:116278
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [2] | |
| References | ||||||
| 1 | Lomefloxacin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Identification of influx transporter for the quinolone antibacterial agent levofloxacin. Mol Pharm. 2007 Jan-Feb;4(1):85-94. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
