Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00597
|
|||||
| Drug Name |
Etravirine
|
|||||
| Synonyms |
4-((6-amino-5-bromo-2-((4-cyanophenyl)amino)-4-pyrimidinyl)oxy)-3,5-dimethyl-benzonitrile; 4-({6-AMINO-5-BROMO-2-[(4-CYANOPHENYL)AMINO]PYRIMIDIN-4-YL}OXY)-3,5-DIMETHYLBENZONITRILE; 4-[6-amino-5-bromo-2-(4-cyanoanilino)pyrimidin-4-yl]oxy-3,5-dimethylbenzonitrile; 65B; DAPY deriv; Diaminopyrimidine deriv; ETR; Etravirine (JAN/USAN/INN); Intelence; Intelence (TN); Intelence(TM); R 165335; R-165335; R165335; R165335-TMC125; TMC 125; TMC-125; TMC-125/R-165335; TMC125
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Human immunodeficiency virus infection [ICD11: 1C62.Z] | Approved | [1] | |||
| Structure |
|
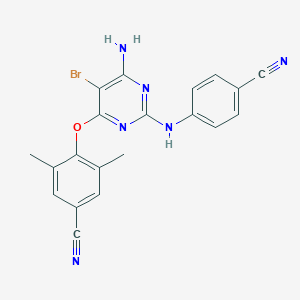 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C20H15BrN6O
|
|||||
| Canonical SMILES |
CC1=CC(=CC(=C1OC2=NC(=NC(=C2Br)N)NC3=CC=C(C=C3)C#N)C)C#N
|
|||||
| InChI |
InChI=1S/C20H15BrN6O/c1-11-7-14(10-23)8-12(2)17(11)28-19-16(21)18(24)26-20(27-19)25-15-5-3-13(9-22)4-6-15/h3-8H,1-2H3,(H3,24,25,26,27)
|
|||||
| InChIKey |
PYGWGZALEOIKDF-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 269055-15-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 435.3 | Topological Polar Surface Area | 121 | ||
| Heavy Atom Count | 28 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
4.5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10261828
, 103268452
, 103999716
, 113457302
, 118844113
, 12015430
, 125346599
, 126617154
, 126632876
, 126665857
, 131308179
, 134224563
, 134338670
, 135171617
, 135611140
, 135693135
, 136946574
, 136980023
, 137006543
, 139263775
, 143497676
, 144115317
, 14856802
, 152035774
, 152234808
, 152258935
, 160647779
, 160658773
, 160968275
, 162009794
, 162011438
, 162200353
, 162849709
, 163894003
, 164831834
, 170483270
, 170503235
, 33514683
, 47206062
, 50755335
, 57397726
, 58108352
, 642445
, 7885445
, 832707
, 92765305
, 92765307
, 93375510
, 93619697
, 99443662
|
|||||
| ChEBI ID |
CHEBI:63589
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MRP3 | Transporter Info | Multidrug resistance-associated protein 3 | Substrate | [2] | |
| References | ||||||
| 1 | Etravirine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Interaction potential of etravirine with drug transporters assessed in vitro. Antimicrob Agents Chemother. 2011 Mar;55(3):1282-4. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
