Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00643
|
|||||
| Drug Name |
Lansoprazole
|
|||||
| Synonyms |
lansoprazole; Prevacid; Monolitum; Bamalite; Lansoprazol; Limpidex; Agopton; Ogastro; Opiren; Lanzor; Prevacid SoluTab; Takepron; Lanzopral; Zoton; Lansoprazolum; Lasoprol; Mesactol; Lansopep; Lanproton; Lancid; Ketian; Prezal; Lanston; Aprazol; Blason; Ulpax; Lanz; Pro Ulco; AG-1749; Compraz; Suprecid; Prosogan; Ilsatec; Promp; Zoprol; Dakar; Prevacid Iv; Ogast; Lanzol-30; AG 1749; Lansoprazol [INN-Spanish]; Lansoprazolum [INN-Latin]; Lansox; Lanzo; A-65006; Prevacid NapraPAC; Prevacid 24HR; Lansoprazole [USAN:BAN:INN]; HSDB 7204
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Peptic ulcer [ICD11: DA61] | Approved | [1] | |||
| Therapeutic Class |
Antiulcer Agents
|
|||||
| Structure |
|
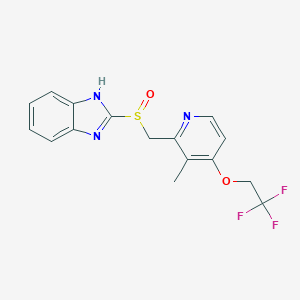 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C16H14F3N3O2S
|
|||||
| Canonical SMILES |
CC1=C(C=CN=C1CS(=O)C2=NC3=CC=CC=C3N2)OCC(F)(F)F
|
|||||
| InChI |
InChI=1S/C16H14F3N3O2S/c1-10-13(20-7-6-14(10)24-9-16(17,18)19)8-25(23)15-21-11-4-2-3-5-12(11)22-15/h2-7H,8-9H2,1H3,(H,21,22)
|
|||||
| InChIKey |
MJIHNNLFOKEZEW-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 103577-45-3
|
|||||
| Pharmaceutical Properties | Molecular Weight | 369.4 | Topological Polar Surface Area | 87.1 | ||
| Heavy Atom Count | 25 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
2.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
104179070
, 104234371
, 126682042
, 128604168
, 135156589
, 137931058
, 140767605
, 14901842
, 15375021
, 162189572
, 164178109
, 164761527
, 170503333
, 175265917
, 175269426
, 175611039
, 178102134
, 184527042
, 185965235
, 210274918
, 210280553
, 223658647
, 223798807
, 226429139
, 23998121
, 241033947
, 251879797
, 252151427
, 252431524
, 252811271
, 25819922
, 44715913
, 80665304
, 89736101
, 96025586
|
|||||
| ChEBI ID |
ChEBI:6375
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Lansoprazole was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | A novel screening strategy to identify ABCB1 substrates and inhibitors. Naunyn Schmiedebergs Arch Pharmacol. 2009 Jan;379(1):11-26. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
