Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00688
|
|||||
| Drug Name |
Gemifloxacin
|
|||||
| Synonyms |
7-(3-Aminomethyl)-4-methoxyimino-pyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid; 7-(3-Aminomethyl-4-methoxyimino-pyrrolidine-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-(1,8)-naphthyridine-3-carboxylic acid; 7-[3-(aminomethyl)-4-(methoxyimino)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid; Factiv; Factiv (TN); Factive; Factive (TN); Gemifloxacin (INN); Gemifloxacin [INN]; Gemifloxacin mesilate; LB 20304; LB 20304a; LB-20304; SB 265805; SB-265805
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Bacterial infections [ICD11: 1A00-1H0Z] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
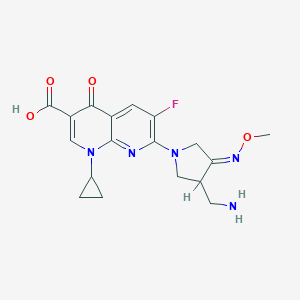
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C18H20FN5O4
|
|||||
| Canonical SMILES |
CON=C1CN(CC1CN)C2=C(C=C3C(=O)C(=CN(C3=N2)C4CC4)C(=O)O)F
|
|||||
| InChI |
InChI=1S/C18H20FN5O4/c1-28-22-14-8-23(6-9(14)5-20)17-13(19)4-11-15(25)12(18(26)27)7-24(10-2-3-10)16(11)21-17/h4,7,9-10H,2-3,5-6,8,20H2,1H3,(H,26,27)/b22-14+
|
|||||
| InChIKey |
ZRCVYEYHRGVLOC-HYARGMPZSA-N
|
|||||
| CAS Number |
CAS 175463-14-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 389.4 | Topological Polar Surface Area | 121 | ||
| Heavy Atom Count | 28 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 10 | |||
| XLogP |
-0.7
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103165376
, 104064190
, 124766444
, 126665045
, 131302500
, 134338130
, 135029930
, 137267488
, 141952322
, 14878468
, 152047100
, 162263733
, 163384560
, 172861476
, 175438039
, 179150073
, 223664023
, 226504227
, 238411063
, 23996711
, 251890177
, 251963973
, 252358754
, 44709303
, 46507905
, 48395332
, 50070532
, 50071315
, 50123382
, 57372939
, 610685
, 79751939
, 85305864
, 85789646
, 91613407
, 96024705
|
|||||
| ChEBI ID |
CHEBI:101853
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [2] | |
| References | ||||||
| 1 | Gemifloxacin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Differential effect of P-gp and MRP2 on cellular translocation of gemifloxacin. Int J Pharm. 2011 Nov 25;420(1):26-33. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
