Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00716
|
|||||
| Drug Name |
Vecuronium
|
|||||
| Synonyms |
(2A,3A,5A,16A,17A)-3,17-diacetoxy-16-(1-methylpiperidinium-1-yl)-2-(piperidin-1-yl)androstane; (2beta,3alpha,5alpha,16beta,17beta)-3,17-diacetoxy-16-(1-methylpiperidinium-1-yl)-2-(piperidin-1-yl)androstane; Norcuron (TN); [(2S,3S,5S,8R,9S,10S,13S,14S,16S,17R)-17-acetyloxy-10,13-dimethyl-16-(1-methylpiperidin-1-ium-1-yl)-2-piperidin-1-yl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl] acetate
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Pain [ICD11: MG30-MG7Z] | Approved | [1] | |||
| Spasms [ICD11: MB47.3] | Approved | [1] | ||||
| Therapeutic Class |
Analgesics
|
|||||
| Structure |
|
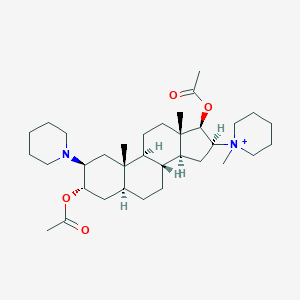 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C34H57N2O4+
|
|||||
| Canonical SMILES |
CC(=O)OC1CC2CCC3C(C2(CC1N4CCCCC4)C)CCC5(C3CC(C5OC(=O)C)[N+]6(CCCCC6)C)C
|
|||||
| InChI |
InChI=1S/C34H57N2O4/c1-23(37)39-31-20-25-12-13-26-27(34(25,4)22-29(31)35-16-8-6-9-17-35)14-15-33(3)28(26)21-30(32(33)40-24(2)38)36(5)18-10-7-11-19-36/h25-32H,6-22H2,1-5H3/q+1/t25-,26+,27-,28-,29-,30-,31-,32-,33-,34-/m0/s1
|
|||||
| InChIKey |
BGSZAXLLHYERSY-XQIGCQGXSA-N
|
|||||
| CAS Number |
CAS 50700-72-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 557.8 | Topological Polar Surface Area | 55.8 | ||
| Heavy Atom Count | 40 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
6.5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
104332628
, 117550021
, 123097011
, 135065904
, 135962408
, 137241739
, 139157515
, 14837446
, 160964634
, 164153588
, 165698506
, 175266821
, 175442172
, 176484500
, 178100828
, 179150509
, 184664916
, 238570034
, 252811085
, 34705625
, 46507656
, 50112721
, 50621251
, 8176218
, 87246434
, 92309078
, 9756
|
|||||
| ChEBI ID |
CHEBI:9939
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Vecuronium was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 2004 Jan;75(1):13-33. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
