Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01281
|
|||||
| Drug Name |
Rizatriptan
|
|||||
| Synonyms |
MK 462 free base; Maxalt (TN); N,N-Dimethyl-2-[5-(1H-1,2,4-triazol-1-ylmethyl)-1H-indol-3-yl]ethylamine; N,N-Dimethyl-5-(1H-1,2,4-triazol-1-ylmethyl)-1H-indole-3-ethanamine; N,N-dimethyl-2-[5-(1,2,4-triazol-1-ylmethyl)-1H-indol-3-yl]-ethanamine; N,N-dimethyl-2-[5-(1,2,4-triazol-1-ylmethyl)-1H-indol-3-yl]ethanamine; N,N-dimethyl-2-[5-(1H-1,2,4-triazol-1-ylmethyl)-1H-indol-3-yl]ethanamine; Risatriptan; Rizaliv (TN); Rizalt (TN); Rizatriptan (INN); Rizatriptan [INN:BAN]; Rizatriptan benzoat; Rizatriptanum
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Migraine Headaches [ICD11: 8A80] | Approved | [1] | |||
| Therapeutic Class |
Antiinflammatory Agents
|
|||||
| Structure |
|
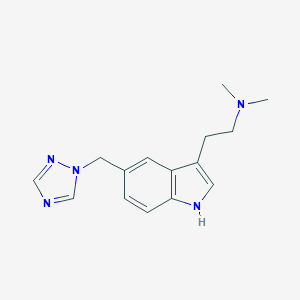 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C15H19N5
|
|||||
| Canonical SMILES |
CN(C)CCC1=CNC2=C1C=C(C=C2)CN3C=NC=N3
|
|||||
| InChI |
InChI=1S/C15H19N5/c1-19(2)6-5-13-8-17-15-4-3-12(7-14(13)15)9-20-11-16-10-18-20/h3-4,7-8,10-11,17H,5-6,9H2,1-2H3
|
|||||
| InChIKey |
ULFRLSNUDGIQQP-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 145202-66-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 269.34 | Topological Polar Surface Area | 49.7 | ||
| Heavy Atom Count | 20 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
1.7
|
|||||
| PubChem CID | ||||||
| PubChem SID |
7380056
, 7980527
, 8153125
, 15221855
, 26612819
, 26749916
, 29224147
, 46506557
, 46530620
, 48416524
, 49830967
, 49984083
, 50173225
, 57322597
, 85209524
, 92718843
, 93166519
, 96025171
, 99373553
, 103236413
, 103941727
, 104308214
, 125001917
, 125728524
, 126621280
, 126658125
, 126667003
, 129384422
, 131299041
, 134337535
, 134358454
, 135110600
, 135650905
, 135684127
, 136375513
, 137002445
, 139157639
, 143494842
, 144205087
, 152036015
, 152239970
, 160964292
, 162011662
, 172091432
, 174007052
, 174477513
, 174527522
, 175266240
, 176484738
, 179116883
|
|||||
| ChEBI ID |
ChEBI:48273
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OCT-1 | Transporter Info | Organic cation transporter 1 | Substrate | [2] | |
| References | ||||||
| 1 | Rizatriptan was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | OCT1 mediates hepatic uptake of sumatriptan and loss-of-function OCT1 polymorphisms affect sumatriptan pharmacokinetics. Clin Pharmacol Ther. 2016 Jun;99(6):633-41. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
