Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01316
|
|||||
| Drug Name |
Cefodizime
|
|||||
| Synonyms |
AC1NSFIB; CDZM; CHEBI:63214; CHEMBL2303613; Cefodizima; Cefodizima [INN-Spanish]; Cefodizime; Cefodizime (INN); Cefodizime Acid; Cefodizime [INN:BAN]; Cefodizimum; Cefodizimum [INN-Latin]; Cefodizme; DTXSID2022757; Diezime; EC 700-301-3; EX-A1379; HR 221 [AS SODIUM]; HR-221; HR-221 [As Sodium]; J-700161; Modivid; Neucef; S-771221B [As Sodium]; SCHEMBL151101; THR 221 [AS SODIUM]; THR-221; THR-221 [As Sodium]; Timecef; UNII-Z31298J4HQ; Z31298J4HQ
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Bacterial infections [ICD11: 1A00-1H0Z] | Approved | [1] | |||
| Therapeutic Class |
Anti-Bacterial Agents
|
|||||
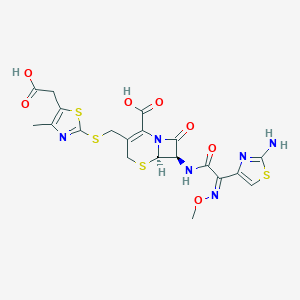
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C20H20N6O7S4
|
|||||
| Canonical SMILES |
CC1=C(SC(=N1)SCC2=C(N3C(C(C3=O)NC(=O)C(=NOC)C4=CSC(=N4)N)SC2)C(=O)O)CC(=O)O
|
|||||
| InChI |
InChI=1S/C20H20N6O7S4/c1-7-10(3-11(27)28)37-20(22-7)36-5-8-4-34-17-13(16(30)26(17)14(8)18(31)32)24-15(29)12(25-33-2)9-6-35-19(21)23-9/h6,13,17H,3-5H2,1-2H3,(H2,21,23)(H,24,29)(H,27,28)(H,31,32)/b25-12-/t13-,17-/m1/s1
|
|||||
| InChIKey |
XDZKBRJLTGRPSS-BGZQYGJUSA-N
|
|||||
| CAS Number |
CAS 69739-16-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 584.7 | Topological Polar Surface Area | 305 | ||
| Heavy Atom Count | 37 | Rotatable Bond Count | 10 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 15 | |||
| XLogP |
0.2
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
CHEBI:63214
|
|||||
| DT(s) Transporting This Drug | MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [2] | |
| References | ||||||
| 1 | The Drugs.com International Drug Name Database: cefodizime | |||||
| 2 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
