Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01322
|
|||||
| Drug Name |
Cilostazol
|
|||||
| Synonyms |
Cilostazole; 3,4-Dihydro-6-(4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy)-2(1H)-quinolinone; 6-(4-(1-Cyclohexyl-1H-tetrazol-5-yl)butoxy)-3,4-dihydro-2(1H)-quinolinone; 6-(4-(1-Cyclohexyl-1H-tetrazol-5-yl)butoxy)-3,4-dihydrocarbostyril; 6-(4-(1-Cyclohexyl-1H-tetrazol-5-yl)butoxyl)-3,4-dihydrocarobostyril; 6-(4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy)-3,4-dihydroquinolin-2(1H)-one; 6-[4-(1-Cyclohexyl-1H-tetrazol-5-yl)-butoxy]-3,4-dihydro-2(1H)-quinolinone; 6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydroquinolin-2(1H)-one; 6-[4-(1-cyclohexyltetrazol-5-yl)butoxy]-3,4-dihydro-1H-quinolin-2-one; 73963-72-1; BRN 3632107; C 0737; C20H27N5O2; CHEBI:31401; CL23867; Cilostazol (JP15/USAN/INN); Cilostazol [INN:JAN]; Cilostazole; Cilostazolum; Cilostazolum [INN-Latin]; MLS000028470; OPC 13013; OPC 21; OPC-13013; OPC-21; Otsuka brand of cilostazol; Pleta (TN); Pletaal; Pletal; Pletal (TN); Pletal, Cilostazol; UNII-N7Z035406B; cilostazol
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Intermittent claudication [ICD11: BD40.00] | Approved | [1] | |||
| Therapeutic Class |
Vasodilator Agents
|
|||||
| Structure |
|
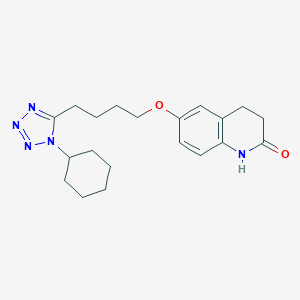 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C20H27N5O2
|
|||||
| Canonical SMILES |
C1CCC(CC1)N2C(=NN=N2)CCCCOC3=CC4=C(C=C3)NC(=O)CC4
|
|||||
| InChI |
InChI=1S/C20H27N5O2/c26-20-12-9-15-14-17(10-11-18(15)21-20)27-13-5-4-8-19-22-23-24-25(19)16-6-2-1-3-7-16/h10-11,14,16H,1-9,12-13H2,(H,21,26)
|
|||||
| InChIKey |
RRGUKTPIGVIEKM-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 73963-72-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 369.5 | Topological Polar Surface Area | 81.9 | ||
| Heavy Atom Count | 27 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
3.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
855683
, 6897916
, 7848958
, 7978527
, 8151779
, 11110906
, 11113335
, 11372358
, 11374323
, 11484992
, 11488934
, 11490985
, 11492587
, 11528739
, 12012700
, 14755273
, 14828756
, 17404781
, 24278291
, 26612838
, 26680450
, 26719653
, 26719654
, 26746958
, 26746959
, 29221909
, 46386747
, 46386994
, 46506317
, 47365365
, 48259412
, 48259413
, 49834949
, 50100194
, 50103999
, 50104000
, 50409718
, 53777324
, 53787948
, 56422089
, 56463107
, 57321435
, 68530811
, 85230957
, 85787626
, 87218800
, 87218801
, 87219014
, 90341176
, 92124839
|
|||||
| ChEBI ID |
CHEBI:29007
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [2] | |
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [3] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [3] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OATP1B1 | Transporter Info | Km = 17.7 microM | Human embryonic kidney cells (HEK293)-OATP1B1 | [3] | |
| OATP1B3 | Transporter Info | Km = 2.7 microM | Human embryonic kidney cells (HEK293)-OATP1B3 | [3] | ||
| References | ||||||
| 1 | Cilostazol was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Aspirin and probenecid inhibit organic anion transporter 3-mediated renal uptake of cilostazol and probenecid induces metabolism of cilostazol in the rat. Drug Metab Dispos. 2014 Jun;42(6):996-1007. | |||||
| 3 | Organic Anion-Transporting Polypeptide and Efflux Transporter-Mediated Hepatic Uptake and Biliary Excretion of Cilostazol and Its Metabolites in Rats and Humans. J Pharm Sci. 2017 Sep;106(9):2515-2523. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
