Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01324
|
|||||
| Drug Name |
Carfilzomib
|
|||||
| Synonyms |
(S)-4-methyl-N-((S)-1-(((S)-4-methyl-1-((R)-2-methyloxiran-2-yl)-1-oxopentan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)-2-((S)-2-(2-morpholinoacetamido)-4-phenylbutanamido)pentanamide; 72X6E3J5AR; 868540-17-4; CHEBI:65347; CHEMBL451887; Carfilzomib; Carfilzomib (PR-171); DSSTox_CID_28616; DSSTox_GSID_48690; DSSTox_RID_82886; Kyprolis; N-{(2S)-2-[(morpholin-4-ylacetyl)amino]-4-phenylbutanoyl}-L-leucyl-N-{(2S)-4-methyl-1-[(2R)-2-methyloxiran-2-yl]-1-oxopentan-2-yl}-L-phenylalaninamide; NCGC00249613-01; PR-171; UNII-72X6E3J5AR
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Multiple myeloma [ICD11: 2A83] | Approved | [1] | |||
| Structure |
|
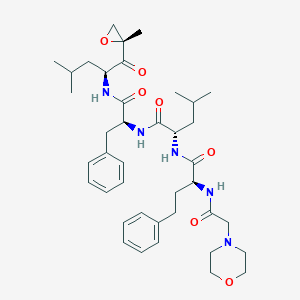 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C40H57N5O7
|
|||||
| Canonical SMILES |
CC(C)CC(C(=O)C1(CO1)C)NC(=O)C(CC2=CC=CC=C2)NC(=O)C(CC(C)C)NC(=O)C(CCC3=CC=CC=C3)NC(=O)CN4CCOCC4
|
|||||
| InChI |
InChI=1S/C40H57N5O7/c1-27(2)22-32(36(47)40(5)26-52-40)42-39(50)34(24-30-14-10-7-11-15-30)44-38(49)33(23-28(3)4)43-37(48)31(17-16-29-12-8-6-9-13-29)41-35(46)25-45-18-20-51-21-19-45/h6-15,27-28,31-34H,16-26H2,1-5H3,(H,41,46)(H,42,50)(H,43,48)(H,44,49)/t31-,32-,33-,34-,40+/m0/s1
|
|||||
| InChIKey |
BLMPQMFVWMYDKT-NZTKNTHTSA-N
|
|||||
| CAS Number |
CAS 868540-17-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 719.9 | Topological Polar Surface Area | 159 | ||
| Heavy Atom Count | 52 | Rotatable Bond Count | 20 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
4.7
|
|||||
| PubChem CID | ||||||
| PubChem SID |
16658822
, 16812122
, 23677375
, 42698607
, 79448448
, 92309411
, 96025563
, 103652542
, 104120353
, 124360326
, 124955654
, 126666807
, 135195928
, 137232018
, 139716850
, 144206630
, 152159713
, 160644888
, 162012022
, 162220763
, 163423918
, 163620703
, 163686020
, 164041848
, 170466878
, 172918803
, 174006371
, 174531085
, 175266683
, 175427135
, 178103992
, 186014508
, 189158922
, 198993058
, 223377621
, 223683622
, 223704692
, 224740444
, 226463473
, 242082461
, 248344031
, 249814475
, 251970960
, 252451821
, 252553657
|
|||||
| ChEBI ID |
ChEBI:65347
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Carfilzomib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Identification of an ABCB1 (P-glycoprotein)-positive carfilzomib-resistant myeloma subpopulation by the pluripotent stem cell fluorescent dye CDy1. Am J Hematol. 2013 Apr;88(4):265-72. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
