Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01331
|
|||||
| Drug Name |
Bortezomib
|
|||||
| Synonyms |
179324-69-7; 69G8BD63PP; Boronic acid,; Bortezomib (PS-341); Bortezomib (Proteasome inhibitor); Bortezomib(JAN/USAN/INN); CHEMBL325041; DPBA; LPD 341; LPD-341; MLN-341; N-[(1R)-1-(DIHYDROXYBORYL)-3-METHYLBUTYL]-N-(PYRAZIN-2-YLCARBONYL)-L-PHENYLALANINAMIDE; N-[(1R)-1-(dihydroxyboranyl)-3-methylbutyl]-Nalpha-(pyrazin-2-ylcarbonyl)-L-phenylalaninamide; PROSCRIPT BORONIC ACID; Peptide boronate; Pyz-Phe-boroLeu; UNII-69G8BD63PP; VELCADE (TN); Velcade; Velcade (TN); Velcade, MG-341, PS-341, Bortezomib; [(1R)-3-Methyl-1-[[(2S)-1-oxo-3-phenyl-2-[(pyrazinylcarbonyl)amino]propyl]amino]butyl]boronic acid; [(1R)-3-methyl-1-[[(2S)-3-phenyl-2-(pyrazine-2-carbonylamino)propanoyl]amino]butyl]boronic acid
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Multiple myeloma [ICD11: 2A83] | Approved | [1] | |||
| Mantle cell lymphoma [ICD11: 2A85.5] | Approved | [1] | ||||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
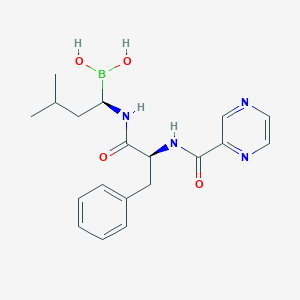 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C19H25BN4O4
|
|||||
| Canonical SMILES |
B(C(CC(C)C)NC(=O)C(CC1=CC=CC=C1)NC(=O)C2=NC=CN=C2)(O)O
|
|||||
| InChI |
InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1
|
|||||
| InChIKey |
GXJABQQUPOEUTA-RDJZCZTQSA-N
|
|||||
| CAS Number |
CAS 179324-69-7
|
|||||
| Pharmaceutical Properties | Molecular Weight | 384.2 | Topological Polar Surface Area | 124 | ||
| Heavy Atom Count | 28 | Rotatable Bond Count | 9 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 6 | |||
| PubChem CID | ||||||
| PubChem SID |
518755
, 600019
, 10276517
, 11433362
, 12015194
, 14853748
, 17137090
, 17397304
, 46508736
, 50726119
, 53787350
, 56311715
, 56311812
, 56312401
, 56313329
, 56314055
, 56314414
, 56314469
, 56314470
, 57402302
, 85096715
, 87325438
, 91611228
, 99432365
, 99443946
, 103338073
, 103963945
, 104603020
, 118048485
, 118318599
, 121360388
, 124756934
, 124950705
, 125163741
, 134340388
, 135696579
, 136023422
, 136342479
, 136367512
, 136920302
, 137030372
, 141858114
, 143497187
, 144206183
, 144206225
, 152049125
, 152239004
, 152344341
, 160829216
, 160968549
|
|||||
| ChEBI ID |
ChEBI:52717
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Bortezomib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | CNS uptake of bortezomib is enhanced by P-glycoprotein inhibition: implications for spinal muscular atrophy. Neurobiol Dis. 2016 Apr;88:118-24. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
