Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01389
|
|||||
| Drug Name |
Tyramine
|
|||||
| Synonyms |
Tyramine; 4-(2-Aminoethyl)phenol; 51-67-2; 4-Hydroxyphenethylamine; p-Tyramine; 2-(4-Hydroxyphenyl)ethylamine; Uteramine; Tyramin; Tyrosamine; Tocosine; 4-Hydroxyphenylethylamine; Systogene; Phenol, 4-(2-aminoethyl)-; p-Hydroxyphenethylamine; Tenosin-wirkstoff; p-Hydroxyphenylethylamine; p-(2-Aminoethyl)phenol; 2-(p-Hydroxyphenyl)ethylamine; Phenethylamine, p-hydroxy-; p-beta-Aminoethylphenol; Phenol, p-(2-aminoethyl)-; Benzeneethanamine, 4-hydroxy-; Tyramine base; beta-Hydroxyphenylethylamine; NSC 249188; p-tyramine; [3H]tyramine
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Parkinson's Disease [ICD11: 8A00.0] | Phase 3 | [1] | |||
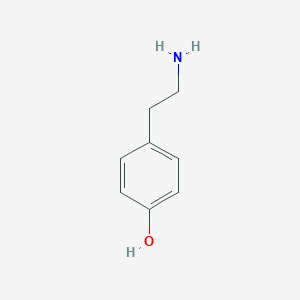
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C8H11NO
|
|||||
| Canonical SMILES |
C1=CC(=CC=C1CCN)O
|
|||||
| InChI |
InChI=1S/C8H11NO/c9-6-5-7-1-3-8(10)4-2-7/h1-4,10H,5-6,9H2
|
|||||
| InChIKey |
DZGWFCGJZKJUFP-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 51-67-2
|
|||||
| Pharmaceutical Properties | Molecular Weight | 137.18 | Topological Polar Surface Area | 46.2 | ||
| Heavy Atom Count | 10 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 2 | |||
| XLogP |
1.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
3766
, 136967
, 608127
, 839997
, 858070
, 3134838
, 5502075
, 8143353
, 8150088
, 8153454
, 10524949
, 11111872
, 11336093
, 11361332
, 11364107
, 11366669
, 11369231
, 11371765
, 11375541
, 11377393
, 11462304
, 11484432
, 11488554
, 11490509
, 11493615
, 11495027
, 15321440
, 24715053
, 24889929
, 24900582
, 25622217
, 26512235
, 26613119
, 26679249
, 26747190
, 26747191
, 26752293
, 26752294
, 29204652
, 29224648
, 41530225
, 47291202
, 47365262
, 47515377
, 47810820
, 47959828
, 47959829
, 48035206
, 49649954
, 49748652
|
|||||
| ChEBI ID |
CHEBI:15760
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OCT-1 | Transporter Info | Organic cation transporter 1 | Substrate | [2] | |
| References | ||||||
| 1 | ClinicalTrials.gov (NCT00203125) A Study to Evaluate the Effects of Tyramine in Patients Who Completed the PRESTO Study. | |||||
| 2 | Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
