Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01392
|
|||||
| Drug Name |
Betamethasone
|
|||||
| Synonyms |
betamethasone; 378-44-9; Betadexamethasone; Flubenisolone; Betamethazone; Rinderon; Visubeta; Celestene; Betnelan; Betapredol; Betacortril; Betacorlan; Methazon; Betsolan; Betamamallet; Hormezon; Betasolon; Betametasone; Cidoten; Bebate; Bedifos; Becort; beta-Methasone; Desacort-Beta; Rinderon A; beta-Methasone alcohol; Betametasona; Betafluorene; Betamethasonum; Celestone; Betamethasone cream; Betamethasone alcohol; Betametasone [DCIT]; 9alpha-Fluoro-16beta-methylprednisolone; Celestone Syrup and Tablets; Celeston; Celestona; Cellestoderm; Luxiqo; Betamethasone Base; Betamethasone Valearate; Betamethasonvalerat Mikron; LT00441022; SCH 4831; Beta-Methasone; Beta-Methasone alcohol; Betametasona [INN-Spanish]; Betamethasonum [INN-Latin]; Betnovate (TN); Celestone (TN); Diprolene (TN); Diprosone (TN); Lotrisone (TN); Rinderon (TN); SCH-4831; Betamethasone (JP15/USP/INN); Betamethasone [USAN:BAN:INN:JAN]; Betamethasone [USAN:INN:BAN:JAN]; Celestone, Betadexamethasone, Flubenisolone, Sch-4831, NCS-39470, Betamethasone; 1,4-Pregnadiene-3,20-dione-9alpha-fluoro-16 beta-methyl-11 beta,17alpha,21-triol; 16beta-Methyl-1,4-pregnadiene-9alpha-fluoro-11beta,17alpha,21-triol-3,20-dione; 9-Fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione; 9-Fluoro-11-beta,17,21-trihydroxy-16-beta-methylpregna-1,4-diene-3,20-dione; 9-Fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione; 9-Fluoro-16beta-methylprednisolone; 9-alpha-Fluoro-16-beta-methylprednisolone; 9alpha-Fluoro-16 beta-methyl-prednisolone; Betamethasone sodium phosphate
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Asthma [ICD11: CA23] | Approved | [1] | |||
| Therapeutic Class |
Antiinflammatory Agents
|
|||||
| Structure |
|
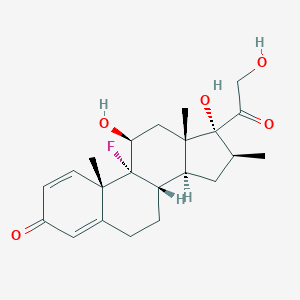 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C22H29FO5
|
|||||
| Canonical SMILES |
CC1CC2C3CCC4=CC(=O)C=CC4(C3(C(CC2(C1(C(=O)CO)O)C)O)F)C
|
|||||
| InChI |
InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15-,16-,17-,19-,20-,21-,22-/m0/s1
|
|||||
| InChIKey |
UREBDLICKHMUKA-DVTGEIKXSA-N
|
|||||
| CAS Number |
CAS 378-44-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 392.5 | Topological Polar Surface Area | 94.8 | ||
| Heavy Atom Count | 28 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
1.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
9066
, 625879
, 3161499
, 7847310
, 7978781
, 8157046
, 10321659
, 11466390
, 11467510
, 11486140
, 12159278
, 14756597
, 15004923
, 24278276
, 24860978
, 24892150
, 26757820
, 29228348
, 46500420
, 46505155
, 46530919
, 47193705
, 47499670
, 47720710
, 47720711
, 47943900
, 48019043
, 48415627
, 49698457
, 50019449
, 50111677
, 53789445
, 56320847
, 56462985
, 57288752
, 57649117
, 57653954
, 75637045
, 85788058
, 87564725
, 87568418
, 92125449
, 93167065
, 103188847
, 103914147
, 104253177
, 104321493
, 121363120
, 124757297
, 124800522
|
|||||
| ChEBI ID |
ChEBI:3077
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Betamethasone was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Structural determinants of P-glycoprotein-mediated transport of glucocorticoids. Pharm Res. 2003 Nov;20(11):1794-803. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
