Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01489
|
|||||
| Drug Name |
Hydroxycamptothecin
|
|||||
| Synonyms |
10-Hydroxycamptothecin; 19685-09-7; (S)-10-Hydroxycamptothecin; Hydroxycamptothecin; 10-hydroxycamptothecine; 10-Hydroxy camptothecin; Hydroxycamptothecine; Camptothecin, hydroxy-; 10-Hydroxy-Camptothecin; (S)-4-Ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione; Camptothecin, 10-hydroxy-; Camptothecine, 10-hydroxy-; UNII-9Z01632KRV; NSC 107124; HCPT; (4S)-4-Ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione; (S)-10-Hydroxycamptothecin hydrate; NSC107124
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Solid tumours [ICD11: 2D4Z] | Phase 1 | [1] | |||
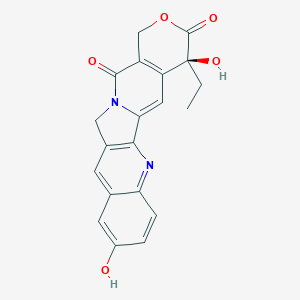
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C20H16N2O5
|
|||||
| Canonical SMILES |
CCC1(C2=C(COC1=O)C(=O)N3CC4=C(C3=C2)N=C5C=CC(=CC5=C4)O)O
|
|||||
| InChI |
InChI=1S/C20H16N2O5/c1-2-20(26)14-7-16-17-11(5-10-6-12(23)3-4-15(10)21-17)8-22(16)18(24)13(14)9-27-19(20)25/h3-7,23,26H,2,8-9H2,1H3/t20-/m0/s1
|
|||||
| InChIKey |
HAWSQZCWOQZXHI-FQEVSTJZSA-N
|
|||||
| CAS Number |
CAS 19685-09-7
|
|||||
| Pharmaceutical Properties | Molecular Weight | 364.4 | Topological Polar Surface Area | 100 | ||
| Heavy Atom Count | 27 | Rotatable Bond Count | 1 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
0.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
407052
, 645392
, 8140019
, 10228777
, 11342034
, 11362217
, 11364533
, 11367095
, 11369657
, 11372600
, 11375589
, 11377819
, 11404568
, 11485555
, 11487619
, 11489476
, 11491436
, 11493629
, 11495453
, 12015125
, 12173672
, 16124509
, 26612627
, 26680421
, 26750003
, 26758893
, 44427566
, 47348452
, 48392929
, 49831587
, 50028362
, 50107786
, 50122774
, 53787862
, 53790463
, 57336414
, 80013607
, 81093235
, 85788508
, 89360248
, 92721280
, 96024160
, 103071286
, 103082955
, 103170545
, 103922002
, 104420324
, 117586316
, 124636619
, 124891994
|
|||||
| ChEBI ID |
CHEBI:81395
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MRP4 | Transporter Info | Multidrug resistance-associated protein 4 | Substrate | [2] | |
| References | ||||||
| 1 | ClinicalTrials.gov (NCT01202370) A Phase I Study of AR-67 (7-t-butyldimethylsilyl-10-hydroxycamptothecin) Given on Days 1, 4 8, 12 & 15 of an Every 21-day Cycle in Adult Patients With Refractory or Metastatic Solid Malignancies | |||||
| 2 | Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
