Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00001
|
|||||
| Drug Name |
Fesoterodine fumarate
|
|||||
| Synonyms |
Toviaz (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Overactive bladder disorder [ICD11: GC50.0] | Approved | [1] | |||
| Structure |
|
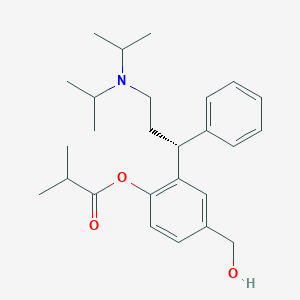 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C26H37NO3
|
|||||
| Canonical SMILES |
CC(C)C(=O)OC1=C(C=C(C=C1)CO)C(CCN(C(C)C)C(C)C)C2=CC=CC=C2
|
|||||
| InChI |
InChI=1S/C26H37NO3/c1-18(2)26(29)30-25-13-12-21(17-28)16-24(25)23(22-10-8-7-9-11-22)14-15-27(19(3)4)20(5)6/h7-13,16,18-20,23,28H,14-15,17H2,1-6H3/t23-/m1/s1
|
|||||
| InChIKey |
DCCSDBARQIPTGU-HSZRJFAPSA-N
|
|||||
| CAS Number |
CAS 286930-03-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 411.6 | Topological Polar Surface Area | 49.8 | ||
| Heavy Atom Count | 30 | Rotatable Bond Count | 11 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 4 | |||
| XLogP |
5.5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
114788164
, 126633314
, 126665680
, 126666536
, 127713256
, 135215466
, 137147467
, 142600549
, 14781939
, 151981129
, 160682498
, 160967872
, 164841556
, 17194959
, 174006830
, 175268192
, 176485080
, 187051776
, 210276627
, 223666877
, 224399961
, 226492628
, 241054700
, 242587777
, 252448583
, 252552193
, 43529928
, 51091564
, 57371974
, 74911026
, 99443256
|
|||||
| ChEBI ID |
ChEBI:135920
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Fesoterodine fumarate was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Blood-brain barrier permeation and efflux exclusion of anticholinergics used in the treatment of overactive bladder. Drugs Aging. 2012 Apr 1;29(4):259-73. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
