Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00026
|
|||||
| Drug Name |
Fexofenadine
|
|||||
| Synonyms |
2-[4-(1-hydroxy-4-{4-[hydroxy(diphenyl)methyl]piperidin-1-yl}butyl)phenyl]-2-methylpropanoic acid; 2-[4-[1-hydroxy-4-[4-[hydroxy(diphenyl)methyl]piperidin-1-yl]butyl]phenyl]-2-methylpropanoic acid; 4-(1-Hydroxy-4-(4-(hydroxydiphenylmethyl)-1-piperidinyl)butyl)-alpha,alpha-dimethylbenzeneacetic acid; Allegra (TN); Carboxyterfenadine; F 9427; Fastofen (TN); Fexofenadine (INN); Fexofenadine [INN:BAN]; Fexofendine; MDL 16455; Telfast (TN); Terfenadine acid metabolite; Terfenadine carboxylate; Terfenadine-COOH; Terfenidine carboxylate, MDL 16455; Tilfur (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Allergic rhinitis [ICD11: CA08.0] | Approved | [1] | |||
| Therapeutic Class |
Antiallergic Agents
|
|||||
| Structure |
|
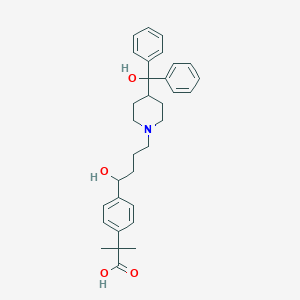 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C32H39NO4
|
|||||
| Canonical SMILES |
CC(C)(C1=CC=C(C=C1)C(CCCN2CCC(CC2)C(C3=CC=CC=C3)(C4=CC=CC=C4)O)O)C(=O)O
|
|||||
| InChI |
InChI=1S/C32H39NO4/c1-31(2,30(35)36)25-17-15-24(16-18-25)29(34)14-9-21-33-22-19-28(20-23-33)32(37,26-10-5-3-6-11-26)27-12-7-4-8-13-27/h3-8,10-13,15-18,28-29,34,37H,9,14,19-23H2,1-2H3,(H,35,36)
|
|||||
| InChIKey |
RWTNPBWLLIMQHL-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 83799-24-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 501.7 | Topological Polar Surface Area | 81 | ||
| Heavy Atom Count | 37 | Rotatable Bond Count | 10 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103238871
, 104303248
, 11364860
, 11367422
, 11369984
, 11372841
, 11373946
, 11378153
, 11484933
, 11488995
, 11491736
, 11492019
, 11495728
, 117867878
, 121361161
, 124749753
, 124880162
, 124880163
, 124891597
, 125727652
, 126525323
, 126683996
, 127278637
, 127278638
, 14835656
, 17405051
, 29222483
, 46504676
, 47953966
, 48029199
, 48029200
, 49846688
, 49878780
, 50105687
, 50105688
, 5309243
, 53777594
, 53787836
, 57321745
, 75782525
, 8152130
, 85164717
, 85209576
, 85231056
, 85789329
, 90341422
, 9212
, 92304031
, 93626300
, 96024653
|
|||||
| ChEBI ID |
ChEBI:5050
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MRP3 | Transporter Info | Multidrug resistance-associated protein 3 | Substrate | [2] | |
| OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [3] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [4] | ||
| OATP2B1 | Transporter Info | Organic anion transporting polypeptide 2B1 | Substrate | [5] | ||
| OCT-1 | Transporter Info | Organic cation transporter 1 | Substrate | [6] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [7] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OATP1A2 | Transporter Info | Km = 6.4 microM | Human cervical cancer cell line (Hela)-OATP1A2 | [8] | |
| OATP1B3 | Transporter Info | Km = 108 microM | Human embryonic kidney cells (HEK293)-OATP1B3 | [4] | ||
| P-GP | Transporter Info | Km = 150 microM | Human enterocyte-like 2 cells (Caco-2)-MDR1 | [9] | ||
| References | ||||||
| 1 | Fexofenadine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Involvement of multiple efflux transporters in hepatic disposition of fexofenadine. Mol Pharmacol. 2008 May;73(5):1474-83. | |||||
| 3 | Influence of the flavonoids apigenin, kaempferol, and quercetin on the function of organic anion transporting polypeptides 1A2 and 2B1. Biochem Pharmacol. 2010 Dec 1;80(11):1746-53. | |||||
| 4 | Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2005 Oct;33(10):1477-81. | |||||
| 5 | The effects of the SLCO2B1 c.1457C>T polymorphism and apple juice on the pharmacokinetics of fexofenadine and midazolam in humans. Pharmacogenet Genomics. 2011 Feb;21(2):84-93. | |||||
| 6 | Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007 Mar;81(3):362-70. | |||||
| 7 | Effect of itraconazole on the pharmacokinetics and pharmacodynamics of fexofenadine in relation to the MDR1 genetic polymorphism. Clin Pharmacol Ther. 2005 Aug;78(2):191-201. | |||||
| 8 | OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999 Aug;27(8):866-71. | |||||
| 9 | Transport characteristics of fexofenadine in the Caco-2 cell model. Pharm Res. 2004 Aug;21(8):1398-404. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
