Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00046
|
|||||
| Drug Name |
Levofloxacin
|
|||||
| Synonyms |
(-)-(S)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid; (-)-(S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyridol[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid hemihydrate; (-)-(S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7Hpyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid hemihydrate; (-)-Ofloxacin; (3S)-9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid; (R)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid; (R)-isomer; (S)-(-)-Ofloxacin; (S)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid; (S)-Ofloxacin; Aeroquin; Cravit; Cravit (TN); Cravit Ophthalmic; D-Levofloxacin; DR 3354; DR-3355; DR-3355: L-isomer of ofloxacin; DR3355; Elequine; Floxacin; Floxel; HR 355; HR-355; Iquix; Iquix (TN); L-Ofloxacin; LEVAQUIN IN DEXTROSE 5% IN PLASTIC CONTAINER; LFX; LVX; Leroxacin; Lesacin; Levaquin; Levaquin (TN); Levofloxacin (INN); Levofloxacin [USAN:INN:JAN]; Levofloxacin tablet, suspension or intravenous; Levofloxacine; Levofloxacine [INN-French];Levofloxacino [INN-Spanish]; Levofloxacino; Levofloxacinum; Levofloxacinum [INN-Latin]; Levokacin; Levox; Levoxacin; MP-376; Mosardal; Nofaxin; Ofloxacin; Ofloxacin S-(-)-form; Oftaquix; Oftaquix (TN); Quixin; Quixin (TN); R-Ofloxacin; RWJ 25213-097; RWJ-25213; Reskuin; S-(-)-Ofloxacin; Tavanic; Tavanic (TN); Volequin
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Gram-positive & negative bacteria infections [ICD11: 1A00-1H0Z] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
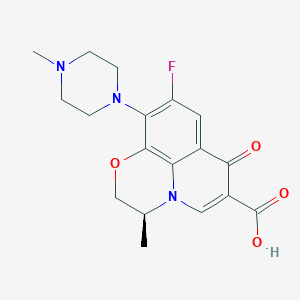 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C18H20FN3O4
|
|||||
| Canonical SMILES |
CC1COC2=C3N1C=C(C(=O)C3=CC(=C2N4CCN(CC4)C)F)C(=O)O
|
|||||
| InChI |
InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)/t10-/m0/s1
|
|||||
| InChIKey |
GSDSWSVVBLHKDQ-JTQLQIEISA-N
|
|||||
| CAS Number |
CAS 100986-85-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 361.4 | Topological Polar Surface Area | 73.3 | ||
| Heavy Atom Count | 26 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
-0.4
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10250227
, 103165325
, 104052737
, 104179033
, 104253435
, 11364613
, 11367175
, 11369737
, 11372008
, 11374743
, 11377899
, 11485627
, 11489492
, 11490810
, 11492937
, 11495533
, 11528725
, 12014081
, 14876841
, 14901428
, 24857060
, 26612693
, 26680408
, 26719895
, 46225907
, 46386771
, 46505134
, 48185231
, 48334772
, 49665952
, 49681682
, 50064059
, 50123181
, 56314311
, 57346857
, 57648297
, 598046
, 76034622
, 7979773
, 85261747
, 85789483
, 87558890
, 89736102
, 92124751
, 92307928
, 92308354
, 92309286
, 92710579
, 96024810
, 9862
|
|||||
| ChEBI ID |
ChEBI:63598
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OATP1A2 | Transporter Info | Km = 136 microM | Oocytes-OATP1A2 | [2] | |
| P-GP | Transporter Info | Km = 5600 microM | Human enterocyte-like 2 cells (Caco-2)-MDR1 | [4] | ||
| P-GP | Transporter Info | Km = 3000 microM | LLC-PK1 cells-MDR1 | [5] | ||
| References | ||||||
| 1 | Levofloxacin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Identification of influx transporter for the quinolone antibacterial agent levofloxacin. Mol Pharm. 2007 Jan-Feb;4(1):85-94. | |||||
| 3 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
| 4 | Secretory mechanisms of grepafloxacin and levofloxacin in the human intestinal cell line caco-2. J Pharmacol Exp Ther. 2000 Oct;295(1):360-6. | |||||
| 5 | Transport of quinolone antibacterial drugs by human P-glycoprotein expressed in a kidney epithelial cell line, LLC-PK1. J Pharmacol Exp Ther. 1997 Aug;282(2):955-60. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
