Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00049
|
|||||
| Drug Name |
Levomilnacipran
|
|||||
| Synonyms |
(1S,2R)-2-(aminomethyl)-N,N-diethyl-1-phenylcyclopropane-1-carboxamide; (1S,2R)-2-(aminomethyl)-N,N-diethyl-1-phenylcyclopropanecarboxamide; 96847-54-0; AC1OCEN8; CHEMBL99946; F 2695; F-2695; Fetzima; Levomilnacipran (USAN/INN); Levomilnacipran HCl; Levomilnacipran [USAN:INN]; Milnacipram; UGM0326TXX; UNII-UGM0326TXX
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Fibromyalgia [ICD11: MG30.01] | Approved | [1] | |||
| Structure |
|
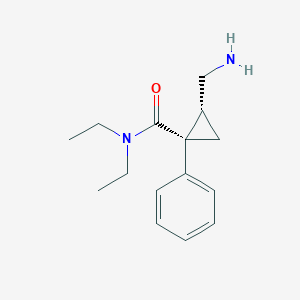 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C15H22N2O
|
|||||
| Canonical SMILES |
CCN(CC)C(=O)C1(CC1CN)C2=CC=CC=C2
|
|||||
| InChI |
InChI=1S/C15H22N2O/c1-3-17(4-2)14(18)15(10-13(15)11-16)12-8-6-5-7-9-12/h5-9,13H,3-4,10-11,16H2,1-2H3/t13-,15+/m0/s1
|
|||||
| InChIKey |
GJJFMKBJSRMPLA-DZGCQCFKSA-N
|
|||||
| CAS Number |
CAS 96847-55-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 246.35 | Topological Polar Surface Area | 46.3 | ||
| Heavy Atom Count | 18 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 2 | |||
| XLogP |
1.4
|
|||||
| PubChem CID | ||||||
| PubChem SID | ||||||
| ChEBI ID |
ChEBI:136040
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Levomilnacipran was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | P-glycoprotein differentially affects escitalopram, levomilnacipran, vilazodone and vortioxetine transport at the mouse blood-brain barrier inivo. Neuropharmacology. 2016 Apr;103:104-11. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
