Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00059
|
|||||
| Drug Name |
Tolvaptan
|
|||||
| Synonyms |
(-)-4'-((7-Chloro-2,3,4,5-tetrahydro-5-hydroxy-1H-1-benzazepin-1-yl)carbonyl)-o-tolu-m-toluidide; 7-Chloro-5-hydroxy-1-(2-methyl-4-(2-methylbenzoylamino)benzoyl)2,3,4,5-tetrahydro-1H-1-benzazepine; Benzazepine derivative, 32; N-[4-(7-chloro-5-hydroxy-2,3,4,5-tetrahydro-1-benzazepine-1-carbonyl)-3-methylphenyl]-2-methylbenzamide; OPC 41061; OPC-41061; Samsca; Samsca (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Euvolaemic hyponatraemia [ICD11: 5C72] | Approved | [1] | |||
| Hypervolaemic hyponatraemia [ICD11: 5C72] | Approved | [1] | ||||
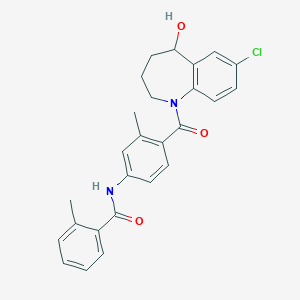
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C26H25ClN2O3
|
|||||
| Canonical SMILES |
CC1=CC=CC=C1C(=O)NC2=CC(=C(C=C2)C(=O)N3CCCC(C4=C3C=CC(=C4)Cl)O)C
|
|||||
| InChI |
InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31)
|
|||||
| InChIKey |
GYHCTFXIZSNGJT-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 150683-30-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 448.9 | Topological Polar Surface Area | 69.6 | ||
| Heavy Atom Count | 32 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
4.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103375036
, 103991970
, 113461194
, 12015154
, 124894207
, 125341597
, 126624049
, 126646501
, 126667032
, 128023819
, 134338710
, 134340568
, 135135410
, 135651270
, 135692541
, 137249406
, 138069831
, 144206807
, 14833208
, 152134154
, 160827363
, 162038188
, 162172255
, 163093186
, 163391946
, 163884836
, 172085105
, 172914598
, 175424760
, 179149970
, 187072445
, 196109765
, 198993114
, 204366097
, 208265503
, 211536279
, 223383397
, 223659992
, 224337427
, 226592757
, 242060063
, 30419982
, 47510146
, 47657166
, 51204548
, 53789150
, 57399753
, 85210210
, 85856516
, 9372812
|
|||||
| ChEBI ID |
CHEBI:32246
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Tolvaptan was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | In vitro P-glycoprotein interactions and steady-state pharmacokinetic interactions between tolvaptan and digoxin in healthy subjects. J Clin Pharmacol. 2011 May;51(5):761-9. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
