Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00063
|
|||||
| Drug Name |
Gemcitabine
|
|||||
| Synonyms |
2',2'-DiF-dC; 2',2'-Difluoro-2'-deoxycytidine; 2',2'-Difluorodeoxycytidine; 2'-Deoxy-.beta.-D-2',2'-difluorocytidine; 2'-Deoxy-2',2'-difluorocytidine; 4-Amino-1-[(2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one; 4-Amino-1-[3,3-difluoro-4-hydroxy-5-(hydroxymethyl) tetrahydrofuran-2-yl]-1H-pyrimidin-2-one; 4-amino-1-((2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)-tetrahydrofuran-2-yl)pyrimidin-2(1H)-one; Cytidine, 2'-deoxy-2',2'-difluoro-2'-Deoxy-.beta.-D-2',2'-difluorocytidine; DDFC; DFdC; DFdCyd; Folfugem; GEO; Gamcitabine; GemLip; Gemcel; Gemcin; Gemcitabina; Gemcitabina [INN-Spanish]; Gemcitabine (USAN/INN); Gemcitabine HCl; Gemcitabine stereoisomer; Gemcitabinum; Gemcitabinum [INN-Latin]; Gemtro; Gemzar; Gemzar (TN); Gemzar (hydrochloride); Inno-D07001; LY 188011; LY-188011; LY188011; Zefei
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Pancreatic cancer [ICD11: 2C10] | Approved | [1] | |||
| Cholangiocarcinoma [ICD11: 2C12.10] | Approved | [1] | ||||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
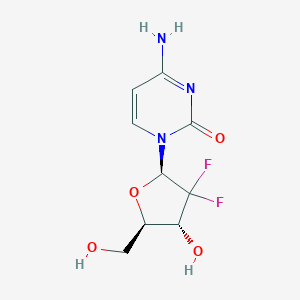 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C9H11F2N3O4
|
|||||
| Canonical SMILES |
C1=CN(C(=O)N=C1N)C2C(C(C(O2)CO)O)(F)F
|
|||||
| InChI |
InChI=1S/C9H11F2N3O4/c10-9(11)6(16)4(3-15)18-7(9)14-2-1-5(12)13-8(14)17/h1-2,4,6-7,15-16H,3H2,(H2,12,13,17)/t4-,6-,7-/m1/s1
|
|||||
| InChIKey |
SDUQYLNIPVEERB-QPPQHZFASA-N
|
|||||
| CAS Number |
CAS 95058-81-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 263.2 | Topological Polar Surface Area | 108 | ||
| Heavy Atom Count | 18 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
-1.5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103233471
, 104321551
, 117682518
, 121264498
, 124893555
, 127340623
, 127340624
, 129221764
, 134338484
, 135022769
, 135684653
, 136367931
, 136369166
, 137001852
, 142312486
, 143493338
, 144116012
, 15197115
, 152059722
, 15221618
, 152240379
, 152258737
, 24769903
, 24875076
, 43118104
, 46506425
, 49684285
, 49835789
, 49960194
, 50298729
, 53787881
, 56311575
, 56312523
, 56312627
, 56312648
, 56313191
, 56313205
, 56313872
, 57304498
, 57314089
, 597200
, 60815357
, 7849427
, 7887820
, 7979381
, 8187035
, 829253
, 87322653
, 92308986
, 9852
|
|||||
| ChEBI ID |
ChEBI:175901
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | CNT1 | Transporter Info | Concentrative nucleoside transporter 1 | Substrate | [2] | |
| MRP5 | Transporter Info | Multidrug resistance-associated protein 5 | Substrate | [3] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [4] | ||
| References | ||||||
| 1 | Gemcitabine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Pancreatic Cancer Chemoresistance to Gemcitabine. Cancers (Basel). 2017 Nov 16;9(11). | |||||
| 3 | Interdependence of gemcitabine treatment, transporter expression, and resistance in human pancreatic carcinoma cells. Neoplasia. 2010 Sep;12(9):740-7. | |||||
| 4 | Increased sensitivity to gemcitabine of P-glycoprotein and multidrug resistance-associated protein-overexpressing human cancer cell lines. Br J Cancer. 2003 Jun 16;88(12):1963-70. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
