Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00066
|
|||||
| Drug Name |
Cidofovir
|
|||||
| Synonyms |
(2S)-3-Hydroxy-2-phosphonylmethoxypropyl-cytosine; (S)-1-(3-Hydroxy-2-phosphonomethoxypropyl)cytosine; (S)-1-[3-hydroxy-2-(phosphonylmethoxy)-propyl]cytosine; (S)-2-(4-Amino-2-oxo-1(2H)-pyrimidinyl-1-(hydroxymethyl)ethoxy)methyl phosphonic acid; (S)-HPMPC; (s)-[[2-(4-amino-2-oxo-1(2h)-pyrimidinyl)-1-(hydroxymethyl)ethoxy]methyl]phosphonic acid; ({[(2S)-1-(4-amino-2-oxopyrimidin-1(2H)-yl)-3-hydroxypropan-2-yl]oxy}methyl)phosphonic acid; 1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine; 1-[(S)-3-Hydroxy-2-(phosphonomethoxy)propyl]-cytosine dihydrate; CDV; Cidofovir (Vistide); Cidofovir (anhydrous); Cidofovir anhydrous; Cidofovirum; Forvade; GS 0504; GS 504; GS-0504; GS-504; GS504; HPMPC; Vistide; Vistide (TN); Vistide, Cidofovir; [(2S)-1-(4-amino-2-oxopyrimidin-1-yl)-3-hydroxypropan-2-yl]oxymethylphosphonic acid
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Cytomegalovirus infections [ICD11: 1D82] | Approved | [1] | |||
| Therapeutic Class |
Anti-HIV Agents
|
|||||
| Structure |
|
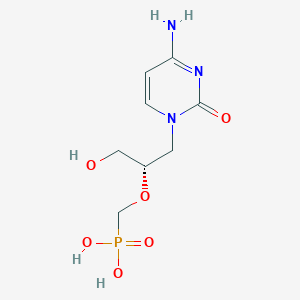 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C8H14N3O6P
|
|||||
| Canonical SMILES |
C1=CN(C(=O)N=C1N)CC(CO)OCP(=O)(O)O
|
|||||
| InChI |
InChI=1S/C8H14N3O6P/c9-7-1-2-11(8(13)10-7)3-6(4-12)17-5-18(14,15)16/h1-2,6,12H,3-5H2,(H2,9,10,13)(H2,14,15,16)/t6-/m0/s1
|
|||||
| InChIKey |
VWFCHDSQECPREK-LURJTMIESA-N
|
|||||
| CAS Number |
CAS 113852-37-2
|
|||||
| Pharmaceutical Properties | Molecular Weight | 279.19 | Topological Polar Surface Area | 146 | ||
| Heavy Atom Count | 18 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
-3.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103208535
, 104253188
, 104321212
, 109692921
, 117588193
, 12014233
, 124757308
, 124896571
, 125164112
, 126628422
, 126655266
, 126664430
, 126669928
, 129535965
, 134222323
, 134338340
, 135018697
, 136920390
, 136946528
, 136949097
, 137005936
, 142388054
, 144115830
, 144206546
, 14848679
, 14972788
, 151994247
, 160963716
, 162011514
, 164778525
, 164807829
, 174007460
, 175268249
, 179149625
, 196106556
, 196403991
, 210275592
, 210281251
, 43117987
, 46506054
, 50007425
, 57314045
, 596838
, 76063703
, 7978941
, 8186963
, 85240541
, 91146456
, 92718864
, 93578310
|
|||||
| ChEBI ID |
CHEBI:3696
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [2] | |
| OAT1 | Transporter Info | Organic anion transporter 1 | Substrate | [3] | ||
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [3] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OAT1 | Transporter Info | Km = 30 microM | Chinese hamster ovary (CHO) cells-OAT1 | [4] | |
| OAT1 | Transporter Info | Km = 58 microM | Chinese hamster ovary (CHO) cells-OAT1 | [5] | ||
| OAT1 | Transporter Info | Km = 46 microM | Oocytes-OAT1 | [6] | ||
| References | ||||||
| 1 | Cidofovir was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
| 3 | Renal transport of adefovir, cidofovir, and tenofovir by SLC22A family members (hOAT1, hOAT3, and hOCT2). Pharm Res. 2007 Apr;24(4):811-5. | |||||
| 4 | Transport of the dipeptidyl peptidase-4 inhibitor sitagliptin by human organic anion transporter 3, organic anion transporting polypeptide 4C1, and multidrug resistance P-glycoprotein. J Pharmacol Exp Ther. 2007 May;321(2):673-83. | |||||
| 5 | Cytotoxicity of antiviral nucleotides adefovir and cidofovir is induced by the expression of human renal organic anion transporter 1. J Am Soc Nephrol. 2000 Mar;11(3):383-93. | |||||
| 6 | The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol Pharmacol. 1999 Sep;56(3):570-80. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
