Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00086
|
|||||
| Drug Name |
L-tryptophan
|
|||||
| Synonyms |
(-)-Tryptophan; (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid; (L)-TRYPTOPHAN; (S)-(-)-2-Amino-3-(3-indolyl)propionic Acid; (S)-(-)-Tryptopha n; (S)-(-)-Tryptophan; (S)-2-Amino-3-(1H-indol-3-yl)propanoic acid; (S)-2-Amino-3-(3-indolyl)propionic acid; (S)-Tryptophan; (S)-a-Amino-1H-indole-3-propanoic acid; (S)-a-Amino-b-indolepropionic acid; (S)-a-Aminoindole-3-propionic acid; (S)-alpha-Amino-1H-indole-3-propanoic acid; (S)-alpha-Amino-beta-indolepropionic acid; (S)-alpha-Aminoindole-3-propionic acid; (S)-alpha-amino-beta-(3-indolyl)-propionic acid; 1-beta-3-Indolylalanine; 151A3008-4CFE-40C9-AC0B-467EF0CB50EA; 1H-Indole-3-alanine; 1H-Indole-3-alanine (VAN); 1beta-3-Indolylalanine; 2-Amino-3-(lH-indol-3-yl)-propanoic acid; 2-Amino-3-indolylpropanoic acid; 2-amino-3-indol-3-ylpropionic acid; 3-Indol-3-ylalanine; Alanine, 3-indol-3-yl; Alpha'-Amino-3-indolepropionic acid; Alpha-Amino-beta-(3-indolyl)-propionic acid; Alpha-amino-beta-(3-indolyl)-pr opionic acid; Alti-Tryptophan; Ardeytropin; EH 121; H-Trp-oh; Indole-3-alanine; Kalma; L-(-)-Tryptophan; L-(-)-Tryptophane; L-TRYPTOPHAN SIGMA GRADE; L-Trp; L-Tryptofan; L-Tryptophan (9CI); L-Tryptophan (JP15); L-Tryptophane; L-Ttp; L-a-Aminoindole-3-propionic acid; L-alpha-Aminoindole-3-propionic acid; L-alpha-amino-3-indolepropionic acid; L-b-3-Indolylalanine; L-beta-3-Indolylalanine; LTR; Lyphan; MT1; Optimax; Pacitron; Propionic acid, 2-amino-3-indol-3-yl; S(-)-1-alpha-Aminoindole-3-propionic acid; Sedanoct; T 0254; TRP NH3+ COOH; TRP-01; Triptofano; Triptofano [Spanish]; Trofan; Trp; Tryptacin; Tryptan; Tryptophan; Tryptophan (H-3); Tryptophan (USP/INN); Tryptophan (VAN); Tryptophan [USAN:INN]; Tryptophan, L-(8CI); Tryptophane; Tryptophane [French]; Tryptophanum; Tryptophanum [Latin]
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Depression [ICD11: 6A8Z] | Approved | [1] | |||
| Therapeutic Class |
Antidepressants
|
|||||
| Structure |
|
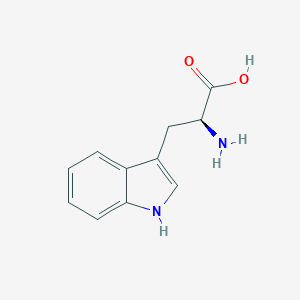 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C11H12N2O2
|
|||||
| Canonical SMILES |
C1=CC=C2C(=C1)C(=CN2)CC(C(=O)O)N
|
|||||
| InChI |
InChI=1S/C11H12N2O2/c12-9(11(14)15)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13H,5,12H2,(H,14,15)/t9-/m0/s1
|
|||||
| InChIKey |
QIVBCDIJIAJPQS-VIFPVBQESA-N
|
|||||
| CAS Number |
CAS 73-22-3
|
|||||
| Pharmaceutical Properties | Molecular Weight | 204.22 | Topological Polar Surface Area | 79.1 | ||
| Heavy Atom Count | 15 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
-1.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10322924
, 10534147
, 11109884
, 11111851
, 11335632
, 11360871
, 11461843
, 11528964
, 14710264
, 14710293
, 14916602
, 15195597
, 17405739
, 24276916
, 24277675
, 24278135
, 24714971
, 24770171
, 24844224
, 24889916
, 24900200
, 24900575
, 25622645
, 26703933
, 26711994
, 26712808
, 3134836
, 3378
, 605298
, 6435732
, 6436701
, 7847088
, 7888703
, 7890877
, 7980851
, 8026883
, 8144649
, 8149576
, 8153976
, 820596
, 821032
, 822330
, 822480
, 823942
, 826461
, 830210
, 834088
, 841615
, 854680
, 855044
|
|||||
| ChEBI ID |
ChEBI:16828
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | LAT2 | Transporter Info | L-type amino acid transporter 2 | Substrate | [2] | |
| MCT10 | Transporter Info | Monocarboxylate transporter 10 | Substrate | [3] | ||
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [4] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [5] | ||
| PAT4 | Transporter Info | Proton-coupled amino acid transporter 4 | Substrate | [6] | ||
| References | ||||||
| 1 | Tryptophan and 5-hydroxytryptophan for depression. Cochrane Database Syst Rev. 2002;(1):CD003198. | |||||
| 2 | The Transporter Classification Database (TCDB): recent advances. Nucleic Acids Res. 2016 Jan 4;44(D1):D372-9. (ID: 2.A.3.8.20) | |||||
| 3 | Phosphatidylserine synthesis required for the maximal tryptophan transport activity in Saccharomyces cerevisiae. Biosci Biotechnol Biochem. 2000 Jan;64(1):167-72. | |||||
| 4 | Murine renal organic anion transporters mOAT1 and mOAT3 facilitate the transport of neuroactive tryptophan metabolites. Am J Physiol Cell Physiol. 2005 Nov;289(5):C1075-84. | |||||
| 5 | Pharmacogenetics of antidepressants. Front Pharmacol. 2011 Feb 16;2:6. | |||||
| 6 | SLC36A4 (hPAT4) is a high affinity amino acid transporter when expressed in Xenopus laevis oocytes. J Biol Chem. 2011 Jan 28;286(4):2455-60. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
