Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00135
|
|||||
| Drug Name |
Colchicine
|
|||||
| Synonyms |
(S)-N-(5,6,7,9-Tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[a]heptalen-7-yl)acetamide; 7-alpha-H-Colchicine; 7.alpha.H-Colchicine; 7alphaH-Colchicine; Benzo(a)heptalen-9(5H)-one; Binds to tubulin; C 9754; Colchicin; Colchicin [German]; Colchicina; Colchicina [Italian]; Colchicine (JP15/USP); Colchicine (TN); Colchicine [JAN]; Colchicine, (+-)-Isomer; Colchicine, (R)-Isomer; Colchicine, Colchicum autumnale; Colchicinum; Colchineos; Colchisol; Colchysat; Colcin; Colcrys; Colsaloid; Colstat; Condylon; Goutnil; Inhibits microtubular assembly; Kolkicin; LOC; MPC-004; N-((7S)-5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo(a)heptalen-7-yl)-acetamide; N-(5,6,7,9-Tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo(a)heptalen-7-yl)acetamide; N-(5,6,7,9-Tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[.alpha.]heptalen-7-yl)-acetamide; N-Acetyl trimethylcolchicinic acid methylether; N-[(7S)-1,2,3,10-tetramethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl]acetamide; N-[(7S)-1,2,3,10-tetramethoxy-9-oxo-6,7-dihydro-5H-benzo[a]heptalen-7-yl]acetamide; N-[(7S)-5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[a]heptalen-7-yl]acetamide; Spindle poison
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Acute gouty arthritis [ICD11: FA25] | Approved | [1] | |||
| Therapeutic Class |
Gout Suppressants
|
|||||
| Structure |
|
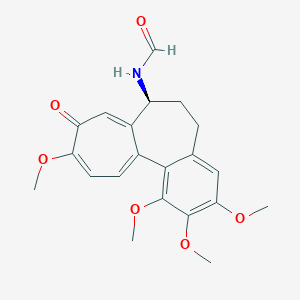 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C21H23NO6
|
|||||
| Canonical SMILES |
COC1=CC=C2C(=CC1=O)C(CCC3=CC(=C(C(=C32)OC)OC)OC)NC=O
|
|||||
| InChI |
InChI=1S/C21H23NO6/c1-25-17-8-6-13-14(10-16(17)24)15(22-11-23)7-5-12-9-18(26-2)20(27-3)21(28-4)19(12)13/h6,8-11,15H,5,7H2,1-4H3,(H,22,23)/t15-/m0/s1
|
|||||
| InChIKey |
HDSXDWASQCHADG-HNNXBMFYSA-N
|
|||||
| CAS Number |
CAS 64-86-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 385.4 | Topological Polar Surface Area | 83.1 | ||
| Heavy Atom Count | 28 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
1
|
|||||
| PubChem CID | ||||||
| PubChem SID | ||||||
| ChEBI ID |
ChEBI:27882
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MRP1 | Transporter Info | Multidrug resistance-associated protein 1 | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | P-GP | Transporter Info | Km = 1.33 microM | CEM/VLB100 cells-MDR1 | [4] | |
| P-GP | Transporter Info | Km = 1640 microM | Human enterocyte-like 2 cells (Caco-2)-MDR1 | [5] | ||
| P-GP | Transporter Info | Km = 45 microM | KB-V1 cells-overexpress MDR1 | [6] | ||
| References | ||||||
| 1 | Colchicine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Pharmacological characterization of the murine and human orthologs of multidrug-resistance protein in transfected human embryonic kidney cells. Mol Pharmacol. 1997 Sep;52(3):344-53. | |||||
| 3 | Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem Pharmacol. 2009 Jul 15;78(2):153-61. | |||||
| 4 | A continuous fluorescence assay for the study of P-glycoprotein-mediated drug efflux using inside-out membrane vesicles. Anal Biochem. 1999 Mar 15;268(2):270-7. | |||||
| 5 | Efflux ratio cannot assess P-glycoprotein-mediated attenuation of absorptive transport: asymmetric effect of P-glycoprotein on absorptive and secretory transport across Caco-2 cell monolayers. Pharm Res. 2003 Aug;20(8):1200-9. | |||||
| 6 | Partial purification and reconstitution of the human multidrug-resistance pump: characterization of the drug-stimulatable ATP hydrolysis. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8472-6. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
