Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00137
|
|||||
| Drug Name |
Nintedanib
|
|||||
| Synonyms |
(Z)-methyl 3-((4-(N-methyl-2-(4-methylpiperazin-1-yl)acetamido)phenylamino)(phenyl)methylene)-2-oxoindoline-6-carboxylate; 1160294-26-7; 656247-17-5; 928326-83-4; BIBF 1120; BIBF-1120; BIBF1120; CHEBI:85164; G6HRD2P839; Intedanib; Methyl (3z)-3-{[(4-{methyl[(4-Methylpiperazin-1-Yl)acetyl]amino}phenyl)amino](Phenyl)methylidene}-2-Oxo-2,3-Dihydro-1h-Indole-6-Carboxylate; Nintedanib (BIBF 1120); OFEV; UNII-G6HRD2P839; Vargatef
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Idiopathic pulmonary fibrosis [ICD11: CB03.4] | Approved | [1] | |||
| Structure |
|
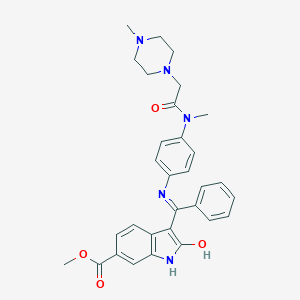 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C31H33N5O4
|
|||||
| Canonical SMILES |
CN1CCN(CC1)CC(=O)N(C)C2=CC=C(C=C2)N=C(C3=CC=CC=C3)C4=C(NC5=C4C=CC(=C5)C(=O)OC)O
|
|||||
| InChI |
InChI=1S/C31H33N5O4/c1-34-15-17-36(18-16-34)20-27(37)35(2)24-12-10-23(11-13-24)32-29(21-7-5-4-6-8-21)28-25-14-9-22(31(39)40-3)19-26(25)33-30(28)38/h4-14,19,33,38H,15-18,20H2,1-3H3
|
|||||
| InChIKey |
CPMDPSXJELVGJG-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 656247-17-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 539.6 | Topological Polar Surface Area | 102 | ||
| Heavy Atom Count | 40 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
4.3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103654502
, 109693020
, 124343350
, 124756932
, 125163739
, 126583666
, 126724182
, 131465103
, 134964383
, 135264167
, 135267499
, 136340157
, 136920292
, 139351111
, 143498980
, 144115708
, 14836969
, 152236441
, 152258852
, 152344119
, 160647702
, 162011437
, 162037383
, 162202550
, 162205188
, 164041723
, 172232574
, 174531531
, 174561006
, 177748905
, 178102559
, 180386833
, 188899496
, 198986794
, 223388548
, 223471427
, 223685460
, 223704774
, 223896861
, 226755113
, 226755114
, 24116377
, 249738349
, 249807126
, 251963000
, 44845702
, 56435863
, 76307381
, 99431587
, 99432362
|
|||||
| ChEBI ID |
ChEBI:85164
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Nintedanib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Effects of Ketoconazole and Rifampicin on the Pharmacokinetics of Nintedanib in Healthy Subjects. Eur J Drug Metab Pharmacokinet. 2018 Oct;43(5):533-541. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
