Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00171
|
|||||
| Drug Name |
Loperamide
|
|||||
| Synonyms |
2-methoxyethyl1-methylethyl2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate; 4-(4-Chlorophenyl)-N,N-dimethyl-.alpha.,.alpha.-diphenyl-4-hydroxy-1-piperidinebutanamide; 4-(4-Chlorophenyl)-N,N-dimethyl-alpha,alpha-diphenyl-4-hydroxy-1-piperidinebutanamide; 4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-N,N-dimethyl-2,2-diphenylbutanamide; Apo-Loperamide; Diamide (TN); Diarr-Eze; Dimor (TN); Imodium (TN); Imodium A-D Caplets; Ioperamide; Kaopectate II; Loperacap; Loperamida; Loperamida [INN-Spanish]; Loperamide (INN); Loperamide Monohydrochloride; Loperamide [INN:BAN]; Loperamidum; Loperamidum [INN-Latin]; Lopex (TN); Maalox Anti-Diarrheal; Nu-Loperamide; PMS-Loperamide; Pepto (TN); Pepto Diarrhea Control; R-18553; Rho-Loperamide
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Acute diarrhea [ICD11: ME05.1] | Approved | [1] | |||
| Therapeutic Class |
Antidiarrheals
|
|||||
| Structure |
|
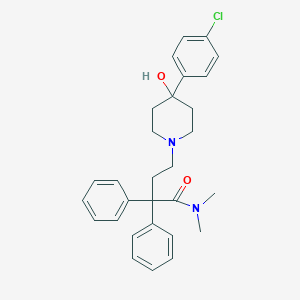 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C29H33ClN2O2
|
|||||
| Canonical SMILES |
CN(C)C(=O)C(CCN1CCC(CC1)(C2=CC=C(C=C2)Cl)O)(C3=CC=CC=C3)C4=CC=CC=C4
|
|||||
| InChI |
InChI=1S/C29H33ClN2O2/c1-31(2)27(33)29(24-9-5-3-6-10-24,25-11-7-4-8-12-25)19-22-32-20-17-28(34,18-21-32)23-13-15-26(30)16-14-23/h3-16,34H,17-22H2,1-2H3
|
|||||
| InChIKey |
RDOIQAHITMMDAJ-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 53179-11-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 477 | Topological Polar Surface Area | 43.8 | ||
| Heavy Atom Count | 34 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10590039
, 11111380
, 11112740
, 11113732
, 11119963
, 11120451
, 11120939
, 11121446
, 11121926
, 11147046
, 11335390
, 11360629
, 11362515
, 11363268
, 11365077
, 11365830
, 11367639
, 11368392
, 11370301
, 11370302
, 11372088
, 11373240
, 11374823
, 11375801
, 11376554
, 11461601
, 11466172
, 11467292
, 11485552
, 11485923
, 11489554
, 11490830
, 11492957
, 11494188
, 14809921
, 26751916
, 26751917
, 29223069
, 46504591
, 47216522
, 47364910
, 47515062
, 47515063
, 47588737
, 47662002
, 47662003
, 5686070
, 7979790
, 8152483
, 9291
|
|||||
| ChEBI ID |
ChEBI:6532
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | P-GP | Transporter Info | Km = 13.8 microM | High five cells-MDR1 | [3] | |
| P-GP | Transporter Info | Km = 11.4 microM | Spodoptera frugiperda (Sf9) cells-MDR1 | [2] | ||
| References | ||||||
| 1 | Loperamide was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | In vitro P-glycoprotein assays to predict the in vivo interactions of P-glycoprotein with drugs in the central nervous system. Drug Metab Dispos. 2008 Feb;36(2):268-75. | |||||
| 3 | Comparative studies on in vitro methods for evaluating in vivo function of MDR1 P-glycoprotein. Pharm Res. 2001 Dec;18(12):1660-8. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
