Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00181
|
|||||
| Drug Name |
Panobinostat
|
|||||
| Synonyms |
(E)-N-HYDROXY-3-(4-{[2-(2-METHYL-1H-INDOL-3-YL)-ETHYLAMINO]-METHYL}-PHENYL)-ACRYLAMIDE; (E)-N-hydroxy-3-[4-[[2-(2-methyl-1H-indol-3-yl)ethylamino]methyl]phenyl]prop-2-enamide; Faridak; LBH 589; LBH-589; LBH-589B; LBH589; NVP-LBH-589; NVP-LBH589; Panobinostat, NVP-LBH589, LBH589
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Multiple myeloma [ICD11: 2A83] | Approved | [1] | |||
| Structure |
|
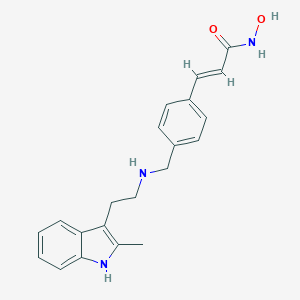 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C21H23N3O2
|
|||||
| Canonical SMILES |
CC1=C(C2=CC=CC=C2N1)CCNCC3=CC=C(C=C3)C=CC(=O)NO
|
|||||
| InChI |
InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+
|
|||||
| InChIKey |
FPOHNWQLNRZRFC-ZHACJKMWSA-N
|
|||||
| CAS Number |
CAS 404950-80-7
|
|||||
| Pharmaceutical Properties | Molecular Weight | 349.4 | Topological Polar Surface Area | 77.2 | ||
| Heavy Atom Count | 26 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103632713
, 109693246
, 114788640
, 118049495
, 12015928
, 123051106
, 124756947
, 125163753
, 126657606
, 126666979
, 126731350
, 127342240
, 127342241
, 131404644
, 131465111
, 134339363
, 134964365
, 135252712
, 136340102
, 136345863
, 136367899
, 136379865
, 136920346
, 137003312
, 140114795
, 143497586
, 144116133
, 152212116
, 152237701
, 152258137
, 152344186
, 15274143
, 160646976
, 162011828
, 162037392
, 162193612
, 163312349
, 164194122
, 164825246
, 17195129
, 172086282
, 43530200
, 57372035
, 62645030
, 75710455
, 81055018
, 87226499
, 87350378
, 93300558
, 99436947
|
|||||
| ChEBI ID |
CHEBI:85990
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Panobinostat was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Panobinostat for the treatment of multiple myeloma: the evidence to date. J Blood Med. 2015 Oct 8;6:269-76. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
