Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00183
|
|||||
| Drug Name |
Tacrine
|
|||||
| Synonyms |
1,2,3,4-TETRAHYDRO-9-ACRIDINAMINE; 1,2,3,4-TETRAHYDRO-9-ACRIDINAMINE (SEE ALSO 1684-40-8); 1,2,3,4-Tetrahydro-9-acridineamine; 1,2,3,4-Tetrahydro-acridin-9-ylamine; 1,2,3,4-Tetrahydroaminoacridine; 1,2,3,4-tetrahydroacridin-9-amine; 5-Amino-6,7,8,9-tetrahydroacridine (European); 9-AMINOTETRAHYDROACRIDINE; 9-Acridinamine, 1,2,3,4-tetrahydro-(9CI); 9-Amino-1,2,3,4-Tetrahydroacridine Hydrate Hydrochloride Hydrate; 9-amino-1,2,3,4-tetrahydroacridine; BBL001044; CS 12602; Cognex; Cognex (TN); Romotal; Tacrina; Tacrina [INN-Spanish]; Tacrinal; Tacrinal (TN); Tacrine (INN); Tacrine [INN:BAN]; Tacrine hydrochloride; Tacrinum; Tacrinum [INN-Latin]; Tenakrin; Tetrahydroaminacrine; Tetrahydroaminoacridine; Tetrahydroaminocrin; Tetrahydroaminocrine; Tha
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Alzheimer disease [ICD11: 8A20] | Approved | [1] | |||
| Therapeutic Class |
Parasympathomimetics
|
|||||
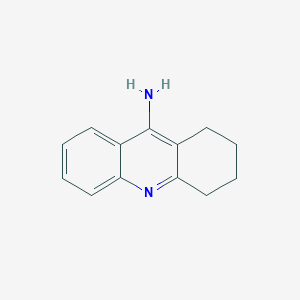
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C13H14N2
|
|||||
| Canonical SMILES |
C1CCC2=NC3=CC=CC=C3C(=C2C1)N
|
|||||
| InChI |
InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15)
|
|||||
| InChIKey |
YLJREFDVOIBQDA-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 321-64-2
|
|||||
| Pharmaceutical Properties | Molecular Weight | 198.26 | Topological Polar Surface Area | 38.9 | ||
| Heavy Atom Count | 15 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 2 | |||
| XLogP |
2.7
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10518487
, 10589113
, 11110734
, 11113824
, 11336111
, 11361350
, 11363310
, 11365872
, 11368434
, 11372617
, 11374476
, 11376596
, 11451154
, 11462322
, 11466357
, 11467477
, 11485559
, 11485994
, 11489628
, 11491524
, 11492634
, 11494230
, 15120928
, 24339135
, 26697094
, 26746578
, 26751997
, 26751998
, 29221124
, 3249865
, 4630
, 46392558
, 46393827
, 46393828
, 46505487
, 46509736
, 47291206
, 47365271
, 47440346
, 47440347
, 47515380
, 47515381
, 47589065
, 47736566
, 47736567
, 597209
, 7423820
, 7890757
, 7980766
, 8151345
|
|||||
| ChEBI ID |
ChEBI:45980
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Tacrine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Tacrine sinusoidal uptake and biliary excretion in sandwich-cultured primary rat hepatocytes. J Pharm Pharm Sci. 2014;17(3):427-38. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
