Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00186
|
|||||
| Drug Name |
Relugolix
|
|||||
| Synonyms |
Relumina; TAK-385
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Endometriosis [ICD11: GA10] | Phase 3 | [1] | |||
| Structure |
|
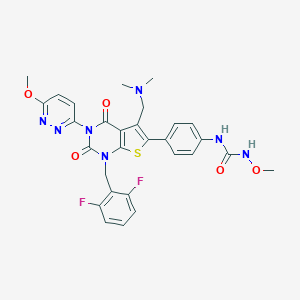 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C29H27F2N7O5S
|
|||||
| Canonical SMILES |
CN(C)CC1=C(SC2=C1C(=O)N(C(=O)N2CC3=C(C=CC=C3F)F)C4=NN=C(C=C4)OC)C5=CC=C(C=C5)NC(=O)NOC
|
|||||
| InChI |
InChI=1S/C29H27F2N7O5S/c1-36(2)14-19-24-26(39)38(22-12-13-23(42-3)34-33-22)29(41)37(15-18-20(30)6-5-7-21(18)31)27(24)44-25(19)16-8-10-17(11-9-16)32-28(40)35-43-4/h5-13H,14-15H2,1-4H3,(H2,32,35,40)
|
|||||
| InChIKey |
AOMXMOCNKJTRQP-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 737789-87-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 623.6 | Topological Polar Surface Area | 158 | ||
| Heavy Atom Count | 44 | Rotatable Bond Count | 9 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 11 | |||
| XLogP |
3.2
|
|||||
| PubChem CID | ||||||
| PubChem SID | ||||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | ClinicalTrials.gov (NCT03931915) Clinical Study to Evaluate Efficacy and Safety of TAK-385 40 mg Compared With Leuprorelin in Patients With Endometriosis | |||||
| 2 | KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (dg:DG01665) | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
