Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00190
|
|||||
| Drug Name |
Amoxicillin
|
|||||
| Synonyms |
(-)-6-(2-Amino-2-(P-hydroxyphenyl)acetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo-(3.2.0)heptane-2-carboxylic acid; (2S,5R,6R)-6-[[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,5R,6R)-6-{[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 6-(2-amino-2-(p-hydroxyphenyl)acetamido)-3,3-dimethyl-7-oxo-, D-(8CI); 6-(D-(-)-alpha-Amino-p-hydroxyphenylacetamido)penicillanic acid; 6-(D-(-)-p-Hydroxy-alpha-aminobenzyl)penicillin; 6-(p-Hydroxy-alpha-aminophenylacetamido)penicillanic acid; 6beta-[(2R)-2-amino-2-(4-hydroxyphenyl)acetamido]-2,2-dimethylpenam-3alpha-carbonyl; 6beta-[(2R)-2-amino-2-(4-hydroxyphenyl)acetamido]-2,2-dimethylpenam-3alpha-carboxylic acid; AMK (TN); AMOXICILLIN CRYSTALLINE; AMOXICILLIN PEDIATRIC; AMPC; Actimoxi; Actimoxi (TN); Alpha-Amino-p-hydroxybenzylpenicillin; Alphamox (TN); Amoclen; Amoksibos (TN); Amoksiklav (TN); Amolin; Amopen; Amopenixin; Amoxi; Amoxi-Mast; Amoxibiotic; Amoxibiotic (TN); Amoxicaps; Amoxicilina; Amoxicilina (TN); Amoxicilina [INN-Spanish]; Amoxicillanyl; Amoxicillin (INN); Amoxicillin (TN); Amoxicillin (anhydrous); Amoxicillin anhydrous; Amoxicilline; Amoxicilline [INN-French]; Amoxicilline [INN]; Amoxicillinum; Amoxicillinum [INN-Latin]; Amoxiclav (TN); Amoxidal (TN); Amoxiden; Amoxil; Amoxil (TN); Amoxin (TN); Amoxivet; Amoxycillin; Amoxycillin Trihydrate; Anemolin; Apo-Amoxi; Apo-Amoxi (TN); Aspenil; Augmentin (TN); BL-P 1410; BLP 1410; BRL-2333; Bactox (TN); Betalaktam (TN); Biomox; Bristamox; Cemoxin; Cilamox (TN); Clamoxyl; Clamoxyl (TN); Curam (TN); D-(-)-alpha-Amino-p-hydroxybenzylpenicillin; D-2-Amino-2-(4-hydroxyphenyl)acetamidopenicillanic acid; D-Amoxicillin; Dedoxil (TN); Delacillin; DisperMox; Dispermox (TN); Duomox (TN); Efpenix; Enhancin (TN); Flemoxin; Geramox (TN); Gimalxina (TN); Hiconcil; Hiconcil (TN); Histocillin; Hydroxyampicillin; Ibiamox; Imacillin; Isimoxin (TN); Klavox (TN); Lamoxy; Lamoxy (TN); Larotid; Metafarma capsules; Metifarma capsules; Moxacin; Moxal; Moxatag; Moxatag (TN); Moxilen (TN); Moxypen (TN); Moxyvit (TN); Nobactam (TN); Novamoxin (TN); Ospamox; Ospamox (TN); P-Hydroxyampicillin; Pamoxicillin; Pamoxicillin (TN); Panamox (TN); Panklav (TN); Piramox; Polymox; Polymox (TN); Ro 10-8756; Robamox; Samthongcillin (TN); Sandoz (TN); Sawamox PM; Senox (TN); Sinacilin (TN); Sumox; Tolodina; Tolodina (TN); Trimox; Trimox (TN); Unicillin; Utimox; Vetramox; Wymox; Wymox (TN); Yucla (TN); Zerrsox (TN); Zimox (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Streptococcal pharyngitis [ICD11: 1B51] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
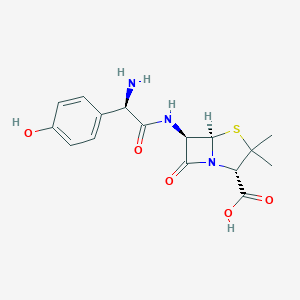 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C16H19N3O5S
|
|||||
| Canonical SMILES |
CC1(C(N2C(S1)C(C2=O)NC(=O)C(C3=CC=C(C=C3)O)N)C(=O)O)C
|
|||||
| InChI |
InChI=1S/C16H19N3O5S/c1-16(2)11(15(23)24)19-13(22)10(14(19)25-16)18-12(21)9(17)7-3-5-8(20)6-4-7/h3-6,9-11,14,20H,17H2,1-2H3,(H,18,21)(H,23,24)/t9-,10-,11+,14-/m1/s1
|
|||||
| InChIKey |
LSQZJLSUYDQPKJ-NJBDSQKTSA-N
|
|||||
| CAS Number |
CAS 26787-78-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 365.4 | Topological Polar Surface Area | 158 | ||
| Heavy Atom Count | 25 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
-2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321668
, 103296891
, 103914155
, 104170168
, 104178892
, 104316487
, 11466385
, 11467505
, 11486110
, 119525230
, 121362635
, 121363090
, 124766400
, 124799877
, 126630772
, 126657267
, 134337739
, 134997708
, 135769296
, 136357150
, 137002906
, 139157548
, 14803689
, 14852747
, 34675405
, 46507578
, 47277019
, 47500930
, 48020429
, 48244812
, 49698452
, 50050650
, 50124271
, 51091783
, 56310905
, 56312972
, 56464141
, 57288747
, 57311544
, 625395
, 75691305
, 7978697
, 8173210
, 85148361
, 855939
, 85787504
, 9045
, 92125419
, 92711519
, 96099939
|
|||||
| ChEBI ID |
ChEBI:2676
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OAT1 | Transporter Info | Organic anion transporter 1 | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [4] | ||
| PEPT2 | Transporter Info | Peptide transporter 2 | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | PEPT2 | Transporter Info | Km = 1040 microM | Madin-Darby canine kidney (MDCK) cells-PEPT2 | [4] | |
| References | ||||||
| 1 | Pantoprazole was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | The anti-influenza drug oseltamivir exhibits low potential to induce pharmacokinetic drug interactions via renal secretion-correlation of in vivo and in vitro studies. Drug Metab Dispos. 2002 Jan;30(1):13-9. | |||||
| 3 | Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007. | |||||
| 4 | Interactions of amoxicillin and cefaclor with human renal organic anion and peptide transporters. Drug Metab Dispos. 2006 Apr;34(4):547-55. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
