Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00222
|
|||||
| Drug Name |
Torsemide
|
|||||
| Synonyms |
1-Isopropyl-3-((4-m-toluidino-3-pyridyl)sulfonyl)urea; 1-[4-(3-methylanilino)pyridin-3-yl]sulfonyl-3-propan-2-ylurea; 1-isopropyl-3-((4-(3-methylphenylamino)pyridine)-3-sulfonyl)urea; 1-isopropyl-3-[(4-m-toluidino-3-pyridyl) sulfonyl]urea; AC 4464; BM 02015; BM-02015; Demadex; Demadex (TN); Demadex, Torsemide; Dilutol; GJ-1090; JDL 464; JDL-464; KS-1123; Luprac; Luprac (TN); N-(((1-Methylethyl)amino)carbonyl)-4-((3-methylphenyl)amino)-3-pyridinesulfonamide; N-(Isopropylcarbamoyl)-4-(m-tolylamino)pyridine-3-sulfonamide; N-{[(1-methylethyl)amino]carbonyl}-4-[(3-methylphenyl)amino]pyridine-3-sulfonamide; PW-2132; Presoril; Sutril; TORSEMIDE; Toradiur; Torasemida; Torasemida [INN-Spanish]; Torasemide (JAN/INN); Torasemide N; Torasemidum; Torasemidum [INN-Latin]; Torem; Torocard; Torrem; Torsemide (USP); Torsemide [USAN]; Unat
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Edema associated with congestive heart failure [ICD11: BD10] | Approved | [1] | |||
| Therapeutic Class |
Diuretics
|
|||||
| Structure |
|
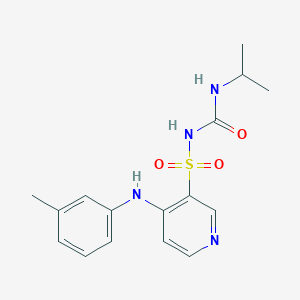 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C16H20N4O3S
|
|||||
| Canonical SMILES |
CC1=CC(=CC=C1)NC2=C(C=NC=C2)S(=O)(=O)NC(=O)NC(C)C
|
|||||
| InChI |
InChI=1S/C16H20N4O3S/c1-11(2)18-16(21)20-24(22,23)15-10-17-8-7-14(15)19-13-6-4-5-12(3)9-13/h4-11H,1-3H3,(H,17,19)(H2,18,20,21)
|
|||||
| InChIKey |
NGBFQHCMQULJNZ-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 56211-40-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 348.4 | Topological Polar Surface Area | 109 | ||
| Heavy Atom Count | 24 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
2.7
|
|||||
| PubChem CID | ||||||
| PubChem SID |
11112793
, 11364670
, 11367232
, 11369794
, 11373102
, 11373959
, 11377957
, 11467058
, 11468178
, 11484799
, 11486816
, 11488958
, 11491647
, 11492105
, 11495590
, 11528723
, 12012656
, 14900728
, 25819958
, 26612831
, 26680347
, 26719820
, 26748988
, 26748989
, 34707389
, 46386585
, 46504760
, 47291390
, 47515595
, 47515596
, 47736767
, 48259511
, 48334790
, 48416649
, 49665992
, 49666477
, 49681707
, 49699258
, 49833537
, 50107501
, 53789088
, 56313713
, 57312605
, 7585786
, 7847448
, 80124867
, 81040892
, 8177279
, 85788916
, 87560438
|
|||||
| ChEBI ID |
CHEBI:9637
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [2] | |
| References | ||||||
| 1 | Torsemide was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Coleman J., Cox A. and Cowley N. (2011). Side Effects of Drugs Annual. Elsevier. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
