Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00224
|
|||||
| Drug Name |
Everolimus
|
|||||
| Synonyms |
(3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34as)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro-9,27-dihydroxy-3-((1R)-2-((1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl)-1-methylethyl)-10,21-dimethoxy-6,8,12,14,20,26-hexamethy; 40-O-(2-hydroxyethyl)-rapamycin; 42-O-(2-Hydroxyethyl)rapamycin; Afinitor; Afinitor (TN); CERTICAN(R); Certican; Certican (TN); Everolimus (JAN/USAN/INN); Everolimus [USAN]; MTOR kinase inhibitors; NVP-RAD-001; RAD 001; RAD-001; RAD-001C; RAD001; RAD001, SDZ-RAD, Certican, Zortress, Afinitor, Everolimus; SDZ-RAD; Zortress
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Renal cell carcinoma [ICD11: 2C90] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
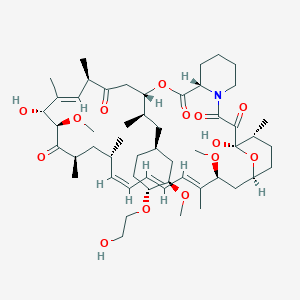 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C53H83NO14
|
|||||
| Canonical SMILES |
CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)OCCO)C)C)O)OC)C)C)C)OC
|
|||||
| InChI |
InChI=1S/C53H83NO14/c1-32-16-12-11-13-17-33(2)44(63-8)30-40-21-19-38(7)53(62,68-40)50(59)51(60)54-23-15-14-18-41(54)52(61)67-45(35(4)28-39-20-22-43(66-25-24-55)46(29-39)64-9)31-42(56)34(3)27-37(6)48(58)49(65-10)47(57)36(5)26-32/h11-13,16-17,27,32,34-36,38-41,43-46,48-49,55,58,62H,14-15,18-26,28-31H2,1-10H3/b13-11+,16-12+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,43-,44+,45+,46-,48-,49+,53-/m1/s1
|
|||||
| InChIKey |
HKVAMNSJSFKALM-GKUWKFKPSA-N
|
|||||
| CAS Number |
CAS 159351-69-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 958.2 | Topological Polar Surface Area | 205 | ||
| Heavy Atom Count | 68 | Rotatable Bond Count | 9 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 14 | |||
| XLogP |
5.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
104178966
, 12014881
, 134338463
, 135156085
, 136340120
, 136929859
, 137140572
, 139754909
, 143493384
, 144206063
, 14767667
, 14865574
, 151990396
, 152236848
, 152258132
, 160645716
, 160646971
, 162189189
, 174527790
, 175265707
, 177748738
, 179150022
, 203355779
, 226396389
, 50044230
, 50112765
, 56311446
, 56312241
, 56312580
, 56313164
, 91613187
|
|||||
| ChEBI ID |
ChEBI:68478
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Everolimus was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Closer to the Site of Action: Everolimus Concentrations in Peripheral Blood Mononuclear Cells Correlate Well With Whole Blood Concentrations. Ther Drug Monit. 2015 Oct;37(5):675-80. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
