Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00228
|
|||||
| Drug Name |
Ceritinib
|
|||||
| Synonyms |
1032900-25-6; 5-Chloro-N2-(5-methyl-4-(piperidin-4-yl)-2-(propan-2-yloxy)phenyl)-N4-(2-(propane-2-sulfonyl)phenyl)pyrim; 5-Chloro-N2-[2-isopropoxy-5-Methyl-4-(4-piperidyl)phenyl]-N4-(2-isopropylsulfonylphenyl)pyriMidine-2,4-diaMine; 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine; AK174337; C28H36ClN5O3S; CHEBI:78432; CHEMBL2403108; Eritinib (LDK378); K418KG2GET; LDK 378; LDK-378; LDK378; LDK378(Ceritinib); NVP-LDK378-NX; UNII-K418KG2GET; ZYKADIA; ceritinib
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Non-small cell lung cancer [ICD11: 2C25] | Approved | [1] | |||
| Structure |
|
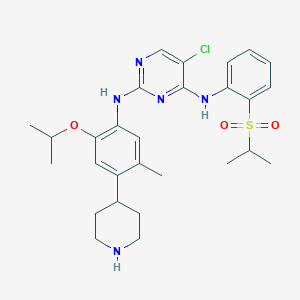 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C28H36ClN5O3S
|
|||||
| Canonical SMILES |
CC1=CC(=C(C=C1C2CCNCC2)OC(C)C)NC3=NC=C(C(=N3)NC4=CC=CC=C4S(=O)(=O)C(C)C)Cl
|
|||||
| InChI |
InChI=1S/C28H36ClN5O3S/c1-17(2)37-25-15-21(20-10-12-30-13-11-20)19(5)14-24(25)33-28-31-16-22(29)27(34-28)32-23-8-6-7-9-26(23)38(35,36)18(3)4/h6-9,14-18,20,30H,10-13H2,1-5H3,(H2,31,32,33,34)
|
|||||
| InChIKey |
VERWOWGGCGHDQE-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 1032900-25-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 558.1 | Topological Polar Surface Area | 114 | ||
| Heavy Atom Count | 38 | Rotatable Bond Count | 9 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
6.4
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
CHEBI:78432
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Ceritinib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | P-glycoprotein Mediates Ceritinib Resistance in Anaplastic Lymphoma Kinase-rearranged Non-small Cell Lung Cancer. EBioMedicine. 2015 Dec 12;3:54-66. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
