Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00233
|
|||||
| Drug Name |
Romidepsin
|
|||||
| Synonyms |
(1S,4S,7Z,10S,16E,21R)-7-Ethylidene-4,21-bis(1-methylethyl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo(8.7.6)tricos-16-ene-3,6,9,19,22-pentone; (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone; (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetrazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone; Antibiotic FR 901228; Chromadax; Chromadax (TN); Cyclo((2Z)-2-amino-2-butenoyl-L-valyl-(3S,4E)-3-hydroxy-7-mercapto-4-heptenoyl-D-valyl-D-cysteinyl), cyclic (35)-disulfide; FK 228; FK-228; FK-901228; FK228; FR 901228; FR-901228; FR901228; HDInhib_000006; Istodax; Istodax (TN); L-Valine, N-((3S,4E)-3-hydroxy-7-mercapto-1-oxo-4-heptenyl)-D-valyl-D-cysteinyl-(2Z)-2-amino-2-butenoxyl-, (4-1)-lactone, cyclic (1-2)-disulfide; Romidepsin (USAN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Cutaneous T-cell lymphoma [ICD11: 2B0Z] | Approved | [1] | |||
| Peripheral T-cell lymphoma [ICD11: 2A90.C] | Approved | [1] | ||||
| Structure |
|
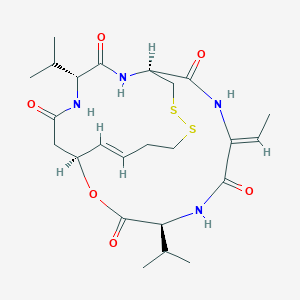 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C24H36N4O6S2
|
|||||
| Canonical SMILES |
CC=C1C(=O)NC(C(=O)OC2CC(=O)NC(C(=O)NC(CSSCCC=C2)C(=O)N1)C(C)C)C(C)C
|
|||||
| InChI |
InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17-,19-,20+/m1/s1
|
|||||
| InChIKey |
OHRURASPPZQGQM-GCCNXGTGSA-N
|
|||||
| CAS Number |
CAS 128517-07-7
|
|||||
| Pharmaceutical Properties | Molecular Weight | 540.7 | Topological Polar Surface Area | 193 | ||
| Heavy Atom Count | 36 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
2.2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103393180
, 104222481
, 113905898
, 11650994
, 12014448
, 127325932
, 127325933
, 131408687
, 134338801
, 134338973
, 134340279
, 137156420
, 139110210
, 14763476
, 14763478
, 152159610
, 163620827
, 163686155
, 164233439
, 175266198
, 176484649
, 178103585
, 184816960
, 198992824
, 224274473
, 226087966
, 226972555
, 251894761
, 252430794
, 252450287
, 252473284
, 35018633
, 46519185
, 47208288
, 47721651
, 47944836
, 48394948
, 494874
, 50062237
, 50260193
, 53788098
, 57361456
, 8139716
, 87226502
|
|||||
| ChEBI ID |
CHEBI:61080
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | ||
| References | ||||||
| 1 | Romidepsin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Population pharmacokinetics of romidepsin in patients with cutaneous T-cell lymphoma and relapsed peripheral T-cell lymphoma. Clin Cancer Res. 2009 Feb 15;15(4):1496-503. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
