Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00253
|
|||||
| Drug Name |
Bosutinib
|
|||||
| Synonyms |
4-((2,4-dichloro-5-methoxyphenyl)amino)-6-methoxy-7-(3-(4-methyl-1-piperazinyl)propoxy)-3-quinolinecarbonitrile; 4-((2,4-dichloro-5-methoxyphenyl)amino)-6-methoxy-7-(3-(4-methylpiperazin-1-yl)propoxy)quinoline-3-carbonitrile; 4-(2,4-dichloro-5-methoxyanilino)-6-methoxy-7-[3-(4-methylpiperazin-1-yl)propoxy]quinoline-3-carbonitrile; 4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl)propo; 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methylpiperazin-1-yl)propoxy]quinoline-3-carbonitrile; Bosutinib (BCR-ABL inhibitor 3rd gen); Bosutinib (USAN); PF-5208763; SKI 606; SKI-606; SKI606; Xy]-3-quinolinecarbonitrile
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Chronic myelogenous leukemia [ICD11: 2A20.0] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
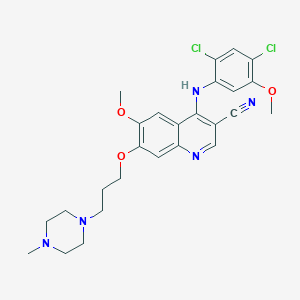
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C26H29Cl2N5O3
|
|||||
| Canonical SMILES |
CN1CCN(CC1)CCCOC2=C(C=C3C(=C2)N=CC(=C3NC4=CC(=C(C=C4Cl)Cl)OC)C#N)OC
|
|||||
| InChI |
InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31)
|
|||||
| InChIKey |
UBPYILGKFZZVDX-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 380843-75-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 530.4 | Topological Polar Surface Area | 82.9 | ||
| Heavy Atom Count | 36 | Rotatable Bond Count | 9 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
5.4
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103220856
, 11061176
, 113911152
, 118844931
, 124950167
, 125011711
, 125349351
, 126627733
, 126647930
, 126666992
, 126731247
, 134339019
, 134964339
, 135246638
, 135642791
, 135684978
, 135685144
, 135685145
, 135685164
, 135697655
, 136340225
, 136368026
, 136920303
, 137127131
, 139943916
, 143495997
, 144115749
, 14720373
, 14885411
, 152041940
, 152234943
, 152258097
, 152344342
, 160646936
, 162011701
, 162170730
, 162201602
, 162256836
, 163884826
, 164041898
, 17397405
, 26676000
, 26746630
, 39301565
, 53641516
, 56459346
, 57361290
, 8034215
, 85246176
, 92721109
|
|||||
| ChEBI ID |
ChEBI:39112
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | |
| References | ||||||
| 1 | Bosutinib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | In vitro and in vivo identification of ABCB1 as an efflux transporter of bosutinib. J Hematol Oncol. 2015 Jul 7;8:81. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
