Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00259
|
|||||
| Drug Name |
Cefaclor
|
|||||
| Synonyms |
(6R,7R)-7-((R)-2-Amino-2-phenylacetamido)-3-chloro-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid monohydrate; (6R,7R)-7-[[(2R)-2-amino-2-phenylacetyl]amino]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(2R)-2-amino-2-phenylacetyl]amino]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid hydrate; (6R,7R)-7-{[(2R)-2-amino-2-phenylacetyl]amino}-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 3-Chloro-7-D-(2-phenylglycinamido)-3-cephem-4-carboxylic acid; 3-Chloro-7-D-(2-phenylglycinamido)-3-cephem-4-carboxylic acid monohydrate; 7-(2-Amino-2-phenyl-acetylamino)-3-chloro-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7beta-{[(2R)-2-amino-2-phenylacetyl]amino}-3-chloro-3,4-didehydrocepham-4-carboxylic acid; Alenfral; Alenfral (TN); Alfacet; Alfatil; Alfatil Kapseln; CCL; Ceclor; Ceclor (TN); Ceclor, Distaclor, Keflor, Raniclor, Cefaclor; Cefaclor (JP15); Cefaclor (USP); Cefaclor [USAN:INN:BAN:JAN]; Cefaclor anhydrous; Cefaclor hydrate; Cefaclor monohydrate; Cefaclor-1-wasser; Cefaclorum; Distaclor; Distaclor (TN); Dystaclor MR; Keflor (TN); Kefolor; Kefolor Suspension; L-Kefral; Lilly 99638 hydrate; Muco Panoral; Panacef; Panoral; Raniclor (TN); S-6472
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Gram-positive & negative bacteria infections [ICD11: 1A00-1H0Z] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
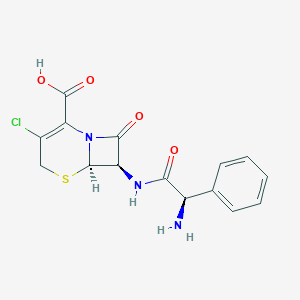 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C15H14ClN3O4S
|
|||||
| Canonical SMILES |
C1C(=C(N2C(S1)C(C2=O)NC(=O)C(C3=CC=CC=C3)N)C(=O)O)Cl
|
|||||
| InChI |
InChI=1S/C15H14ClN3O4S/c16-8-6-24-14-10(13(21)19(14)11(8)15(22)23)18-12(20)9(17)7-4-2-1-3-5-7/h1-5,9-10,14H,6,17H2,(H,18,20)(H,22,23)/t9-,10-,14-/m1/s1
|
|||||
| InChIKey |
QYIYFLOTGYLRGG-GPCCPHFNSA-N
|
|||||
| CAS Number |
CAS 53994-73-3
|
|||||
| Pharmaceutical Properties | Molecular Weight | 367.8 | Topological Polar Surface Area | 138 | ||
| Heavy Atom Count | 24 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
-1.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
11342218
, 11362401
, 11363964
, 11366526
, 11369088
, 11372875
, 11373525
, 11377250
, 11466513
, 11467130
, 11467633
, 11468250
, 11484973
, 11486006
, 11486818
, 11487803
, 11489005
, 11491673
, 11491908
, 11494884
, 12013565
, 14803830
, 14852885
, 24278331
, 26612067
, 26680440
, 29215008
, 34715524
, 46386958
, 46507693
, 47425324
, 47425325
, 47574401
, 47647541
, 47796074
, 48020027
, 48095565
, 48170475
, 48244405
, 48244406
, 48415708
, 48484011
, 49681804
, 49698517
, 625389
, 7847322
, 7978871
, 8181891
, 855765
, 9094
|
|||||
| ChEBI ID |
CHEBI:3478
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OAT1 | Transporter Info | Organic anion transporter 1 | Substrate | [2] | |
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [3] | ||
| PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [4] | ||
| PEPT2 | Transporter Info | Peptide transporter 2 | Substrate | [5] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | PEPT2 | Transporter Info | Km = 72 microM | Madin-Darby canine kidney (MDCK) cells-PEPT2 | [5] | |
| References | ||||||
| 1 | Cefaclor was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | FDA Drug Development and Drug Interactions | |||||
| 3 | Human organic anion transporter hOAT3 is a potent transporter of cephalosporin antibiotics, in comparison with hOAT1. Biochem Pharmacol. 2005 Oct 1;70(7):1104-13. | |||||
| 4 | Protein hydrolysate-induced cholecystokinin secretion from enteroendocrine cells is indirectly mediated by the intestinal oligopeptide transporter PepT1. Am J Physiol Gastrointest Liver Physiol. 2011 May;300(5):G895-902. | |||||
| 5 | Interactions of amoxicillin and cefaclor with human renal organic anion and peptide transporters. Drug Metab Dispos. 2006 Apr;34(4):547-55. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
