Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00263
|
|||||
| Drug Name |
Nizatidine
|
|||||
| Synonyms |
(E)-1-N'-[2-[[2-(dimethylaminomethyl)-1,3-thiazol-4-yl]methylsulfanyl]ethyl]-1-N-methyl-2-nitroethene-1,1-diamine; (E)-N-[2-[[2-(dimethylaminomethyl)-1,3-thiazol-4-yl]methylsulfanyl]ethyl]-N'-methyl-2-nitroethene-1,1-diamine; (E)-N-{2-[({2-[(dimethylamino)methyl]-1,3-thiazol-4-yl}methyl)thio]ethyl}-N'-methyl-2-nitroethene-1,1-diamine; Acinon; Acinon (TN); Antizid; Axid; Axid (TN); Axid Ar; Calmaxid; Cronizat; Distaxid; Galitidin; Gastrax; LY 139037; LY-139037; N-(2-(((2-((Dimethylamino)methyl)-4-thiazolyl)methyl)thio)ethyl)-N'-methyl-2-nitro-1,1-ethenediamine; N-(4-(6-Methylamino-7-nitro-2-thia-5-aza-6-hepten-1-yl)-2-thiazolylmethyl)-N,N-dimethylamin; Naxidine; Niatidine; Nizatidina; Nizatidina [Spanish]; Nizatidine (JAN/USP/INN); Nizatidine [USAN:BAN:INN:JAN]; Nizatidinum; Nizatidinum [Latin]; Nizax; Nizaxid; Panaxid; Splendil ER; Tazac; Tazac (TN); Ulcosol; Ulxid; ZE-101; ZL-101; Zanizal; Zinga
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Acid reflux disorder [ICD11: DA22] | Approved | [1] | |||
| Therapeutic Class |
Antiulcer Agents
|
|||||
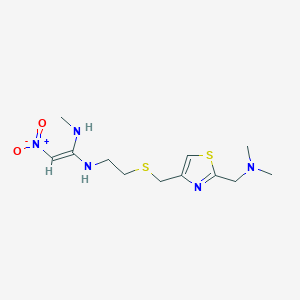
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C12H21N5O2S2
|
|||||
| Canonical SMILES |
CNC(=C[N+](=O)[O-])NCCSCC1=CSC(=N1)CN(C)C
|
|||||
| InChI |
InChI=1S/C12H21N5O2S2/c1-13-11(6-17(18)19)14-4-5-20-8-10-9-21-12(15-10)7-16(2)3/h6,9,13-14H,4-5,7-8H2,1-3H3/b11-6+
|
|||||
| InChIKey |
SGXXNSQHWDMGGP-IZZDOVSWSA-N
|
|||||
| CAS Number |
CAS 76963-41-2
|
|||||
| Pharmaceutical Properties | Molecular Weight | 331.5 | Topological Polar Surface Area | 140 | ||
| Heavy Atom Count | 21 | Rotatable Bond Count | 9 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
1.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10047729
, 103091650
, 103157346
, 103189241
, 11112849
, 111634652
, 117377111
, 117480196
, 119526527
, 12012865
, 124658842
, 124801333
, 124882685
, 126630925
, 131269073
, 131326051
, 134337651
, 135011516
, 135692320
, 137005454
, 142971063
, 144075999
, 144089140
, 14801956
, 152040062
, 160963930
, 172080209
, 177748315
, 178103822
, 179116550
, 179148990
, 26719892
, 36077514
, 46386765
, 46507554
, 48395159
, 48416335
, 49648463
, 49968698
, 57352747
, 7847506
, 7980136
, 80039644
, 85175204
, 85209480
, 92308159
, 92308434
, 92711372
, 93166919
, 9479
|
|||||
| ChEBI ID |
ChEBI:7601
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OCT-1 | Transporter Info | Organic cation transporter 1 | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Nizatidine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem. 2013 Sep 26;56(18):7232-42. | |||||
| 3 | DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018 Jan 4;46(D1):D1074-D1082. (ID: DB00585) | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
