Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00270
|
|||||
| Drug Name |
Vigabatrin
|
|||||
| Synonyms |
(R,S)-4-Amino-5-hexenoic acid; (inverted question mark)-gamma-Vinyl GABA; 4-Amino-5-hexenoic acid; 4-Aminohexenoic acid; 4-aminohex-5-enoic acid; Acid, gamma-Vinyl-gamma-Aminobutyric; Aventis Brand of Vigabatrin; CPP-109; GVG; Gamma Vinyl GABA; Gamma Vinyl gamma Aminobutyric Acid; Gamma-Vinyl GABA; Gamma-Vinyl-GABA; Gamma-Vinyl-gamma-Aminobutyric Acid; Hexenoic acid, 4-amino; Hoechst Brand of Vigabatrin; M071754; MDL 71,754; MDL 71754; MDL-71754; RMI 71754; RMI-71754; RMI-71890; Sabril; Sabril (TN); Sabrilex; Sabrilex (TN); V 8261; V8261_SIGMA; Vigabatrin (JAN/USAN/INN); Vigabatrin Aventis Brand; Vigabatrin Hoechst Brand; Vigabatrin Yamanouchi Brand; Vigabatrin [USAN:BAN:INN]; Vigabatrin [USAN:INN:BAN]; Vigabatrina; Vigabatrina [Spanish]; Vigabatrine; Vigabatrine [French]; Vigabatrinum; Vigabatrinum [Latin]; Yamanouchi Brand of Vigabatrin
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Complex partial seizure [ICD11: 8A6Z] | Approved | [1] | |||
| Epilepsy [ICD11: 8A6Z] | Approved | [1] | ||||
| Infantile spasm [ICD11: 8A62.0] | Approved | [1] | ||||
| Therapeutic Class |
Anticonvulsants
|
|||||
| Structure |
|
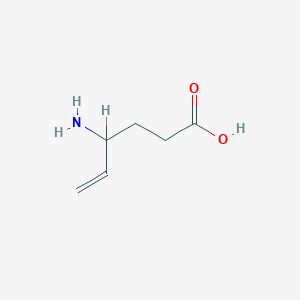 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C6H11NO2
|
|||||
| Canonical SMILES |
C=CC(CCC(=O)O)N
|
|||||
| InChI |
InChI=1S/C6H11NO2/c1-2-5(7)3-4-6(8)9/h2,5H,1,3-4,7H2,(H,8,9)
|
|||||
| InChIKey |
PJDFLNIOAUIZSL-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 60643-86-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 129.16 | Topological Polar Surface Area | 63.3 | ||
| Heavy Atom Count | 9 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
-2.2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321780
, 11341935
, 11362118
, 11363262
, 11365824
, 11368386
, 11373068
, 11376548
, 11466529
, 11467649
, 11486078
, 11487520
, 11491640
, 11494182
, 12012774
, 15194489
, 17405831
, 24278202
, 26612272
, 26747129
, 26751903
, 29224702
, 46507052
, 47217077
, 47662561
, 47960037
, 47960038
, 47960039
, 48035425
, 48334803
, 48334804
, 49698839
, 49976606
, 50104581
, 50104582
, 50104583
, 53778383
, 53788300
, 5440157
, 56313009
, 57322887
, 634838
, 7847601
, 79363997
, 7980880
, 8153478
, 85231284
, 90340732
, 92125387
, 9703
|
|||||
| ChEBI ID |
CHEBI:63638
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | PAT1 | Transporter Info | Proton-coupled amino acid transporter 1 | Substrate | [2] | |
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | PAT1 | Transporter Info | Km = 5200 microM | X.Laevis oocytes-hPAT1 | [2] | |
| References | ||||||
| 1 | Vigabatrin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Rectal absorption of vigabatrin, a substrate of the proton coupled amino acid transporter (PAT1, Slc36a1), in rats. Pharm Res. 2012 Apr;29(4):1134-42. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
