Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00272
|
|||||
| Drug Name |
Bumetanide
|
|||||
| Synonyms |
3-(Aminosulfonyl)-5-(butylamino)-4-phenoxybenzoic acid; 3-(Butylamino)-4-phenoxy-5-sulfamoylbenzoic acid; 3-(aminosulfonyl)-5-(butylamino)-4-(phenyloxy)benzoic acid; 3-butylamino-4-(phenoxy)-5-sulfamoylbenzoic acid; Aquazone; AstraZeneca Brand of Bumetanide; Atlantis Brand of Bumetanide; B 3023; Bumedyl; Bumetanida; Bumetanida [INN-Spanish]; Bumetanide (JP15/USP); Bumetanide (JP15/USP/INN); Bumetanide AstraZeneca Brand; Bumetanide Atlantis Brand; Bumetanide Farmacusi Brand; Bumetanide Grossmann Brand; Bumetanide Leo Brand; Bumetanide Roche Brand; Bumetanide Senosiain Brand; Bumetanide [USAN:BAN:INN:JAN]; Bumetanidum; Bumetanidum [INN-Latin]; Bumethanide; Bumex; Bumex (TN); Bumex, Bumetanide; Burine; Burinex; Butinat; Cambiex; Diurama; Drenural; Farmacusi Brand of Bumetanide; Fontego; Fordiuran; Grossmann Brand of Bumetanide; Leo Brand of Bumetanide; Lixil; Lixil-Leo; Lunetoron; Miccil; PF 1593; PF-1593; PF1593; Ro 10-6338; Ro-10-6338; Roche Brand of Bumetanide; Segurex; Senosiain Brand of Bumetanide; Yurinex
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Edema associated with congestive heart failure [ICD11: BD10] | Approved | [1] | |||
| Therapeutic Class |
Diuretics
|
|||||
| Structure |
|
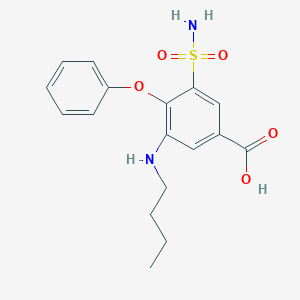 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C17H20N2O5S
|
|||||
| Canonical SMILES |
CCCCNC1=C(C(=CC(=C1)C(=O)O)S(=O)(=O)N)OC2=CC=CC=C2
|
|||||
| InChI |
InChI=1S/C17H20N2O5S/c1-2-3-9-19-14-10-12(17(20)21)11-15(25(18,22)23)16(14)24-13-7-5-4-6-8-13/h4-8,10-11,19H,2-3,9H2,1H3,(H,20,21)(H2,18,22,23)
|
|||||
| InChIKey |
MAEIEVLCKWDQJH-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 28395-03-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 364.4 | Topological Polar Surface Area | 127 | ||
| Heavy Atom Count | 25 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
2.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321639
, 11110843
, 11110844
, 11120301
, 11120789
, 11121277
, 11121573
, 11122053
, 11335209
, 11360448
, 11362642
, 11364211
, 11365204
, 11366773
, 11367766
, 11369335
, 11370555
, 11370556
, 11372660
, 11373367
, 11373578
, 11375928
, 11377497
, 11461420
, 11466304
, 11467424
, 11484716
, 11486107
, 11488866
, 11491296
, 11491845
, 11495131
, 14852701
, 17404725
, 24278265
, 26612248
, 26680422
, 26746951
, 26746952
, 26751481
, 26758346
, 29221635
, 46508147
, 4652638
, 47216571
, 47440017
, 7847313
, 8149787
, 8151648
, 855675
|
|||||
| ChEBI ID |
CHEBI:3213
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MCT6 | Transporter Info | Monocarboxylate transporter 6 | Substrate | [2] | |
| NCC | Transporter Info | Thiazide-sensitive sodium-chloride cotransporter | Substrate | [3] | ||
| OAT2 | Transporter Info | Organic anion transporter 2 | Substrate | [4] | ||
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [5] | ||
| OAT4 | Transporter Info | Organic anion transporter 4 | Substrate | [5] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OAT2 | Transporter Info | Km = 7.52 microM | Oocytes-OAT2 | [4] | |
| OAT3 | Transporter Info | Km = 1586 microM | Proximal tubule (S2) cells-OAT3 | [5] | ||
| OAT4 | Transporter Info | Km = 306 microM | Proximal tubule (S2) cells-OAT4 | [5] | ||
| References | ||||||
| 1 | Bumetanide was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Quercetin, Morin, Luteolin, and Phloretin Are Dietary Flavonoid Inhibitors of Monocarboxylate Transporter 6. Mol Pharm. 2017 Sep 5;14(9):2930-2936. | |||||
| 3 | Genetic variation in the renal sodium transporters NKCC2, NCC, and ENaC in relation to the effects of loop diuretic drugs. Clin Pharmacol Ther. 2007 Sep;82(3):300-9. | |||||
| 4 | Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]). J Pharm Pharmacol. 2005 May;57(5):573-8. | |||||
| 5 | Interactions of human organic anion transporters with diuretics. J Pharmacol Exp Ther. 2004 Mar;308(3):1021-9. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
